Synthesis of 10- O-aryl-substituted berberine derivatives by Chan-Evans-Lam coupling and investigation of their DNA-binding properties
- PMID: 34025807
- PMCID: PMC8111429
- DOI: 10.3762/bjoc.17.81
Synthesis of 10- O-aryl-substituted berberine derivatives by Chan-Evans-Lam coupling and investigation of their DNA-binding properties
Abstract
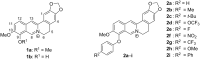
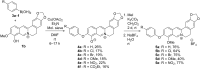
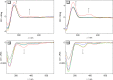
Eleven novel 10-O-aryl-substituted berberrubine and berberine derivatives were synthesized by the Cu2+-catalyzed Chan-Evans-Lam coupling of berberrubine with arylboronic acids and subsequent 9-O-methylation. The reaction is likely introduced by the Cu2+-induced demethylation of berberrubine and subsequent arylation of the resulting 10-oxyanion functionality. Thus, this synthetic route represents the first successful Cu-mediated coupling reaction of berberine substrates. The DNA-binding properties of the 10-O-arylberberine derivatives with duplex and quadruplex DNA were studied by thermal DNA denaturation experiments, spectrometric titrations as well as CD and LD spectroscopy. Fluorimetric DNA melting analysis with different types of quadruplex DNA revealed a moderate stabilization of the telomeric quadruplex-forming oligonucleotide sequence G3(TTAG3)3. The derivatives showed a moderate affinity towards quadruplex DNA (K b = 5-9 × 105 M-1) and ct DNA (K b = 3-5 × 104 M-1) and exhibited a fluorescence light-up effect upon complexation to both DNA forms, with slightly higher intensity in the presence of the quadruplex DNA. Furthermore, the CD- and LD-spectroscopic studies revealed that the title compounds intercalate into ct DNA and bind to G4-DNA by terminal stacking.
Keywords: Cu-mediated coupling reactions; DNA recognition; berberine alkaloids; nucleic acids; quadruplex DNA.
Copyright © 2021, Wickhorst et al.
Figures




Similar articles
-
Selective, pH-Dependent Colorimetric and Fluorimetric Detection of Quadruplex DNA with 4-Dimethylamino(phenyl)-Substituted Berberine Derivatives.Chemistry. 2021 Jun 10;27(33):8580-8589. doi: 10.1002/chem.202100297. Epub 2021 May 5. Chemistry. 2021. PMID: 33855748 Free PMC article.
-
Synthesis and investigation of quadruplex-DNA-binding, 9-O-substituted berberine derivatives.Beilstein J Org Chem. 2020 Nov 18;16:2795-2806. doi: 10.3762/bjoc.16.230. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 33281983 Free PMC article.
-
Berberrubine Phosphate: A Selective Fluorescent Probe for Quadruplex DNA.Molecules. 2021 Apr 28;26(9):2566. doi: 10.3390/molecules26092566. Molecules. 2021. PMID: 33924894 Free PMC article.
-
Synthetic applications and methodology development of Chan-Lam coupling: a review.Mol Divers. 2019 Feb;23(1):215-259. doi: 10.1007/s11030-018-9870-z. Epub 2018 Aug 30. Mol Divers. 2019. PMID: 30159807 Review.
-
Recent Developments in Arylation of N-Nucleophiles via Chan-Lam Reaction: Updates from 2012 Onwards.Curr Org Synth. 2022;19(1):16-30. doi: 10.2174/1570179417666210105120706. Curr Org Synth. 2022. PMID: 33402088 Review.
Cited by
-
Structural modifications of berberine and their binding effects towards polymorphic deoxyribonucleic acid structures: A review.Front Pharmacol. 2022 Aug 9;13:940282. doi: 10.3389/fphar.2022.940282. eCollection 2022. Front Pharmacol. 2022. PMID: 36016553 Free PMC article. Review.
References
-
- Domingo M P, Pardo J, Cebolla V, Galvez E M. Mini-Rev Org Chem. 2010;7:335–340. doi: 10.2174/157019310792246445. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
