Nitroalkene reduction in deep eutectic solvents promoted by BH3NH3
- PMID: 34025809
- PMCID: PMC8111428
- DOI: 10.3762/bjoc.17.83
Nitroalkene reduction in deep eutectic solvents promoted by BH3NH3
Abstract
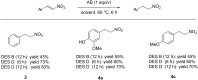
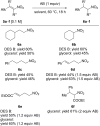
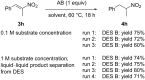
Deep eutectic solvents (DESs) have gained attention as green and safe as well as economically and environmentally sustainable alternative to the traditional organic solvents. Here, we report the combination of an atom-economic, very convenient and inexpensive reagent, such as BH3NH3, with bio-based eutectic mixtures as biorenewable solvents in the synthesis of nitroalkanes, valuable precursors of amines. A variety of nitrostyrenes and alkyl-substituted nitroalkenes, including α- and β-substituted nitroolefins, were chemoselectively reduced to the nitroalkanes, with an atom economy-oriented, simple and convenient experimental procedure. A reliable and easily reproducible protocol to isolate the product without the use of any organic solvent was established, and the recyclability of the DES mixture was successfully investigated.
Keywords: DES; alternative solvents; atom economy; nitro derivatives; reduction.
Copyright © 2021, Faverio et al.
Figures
References
-
- Alonso D A, Baeza A, Chinchilla R, Guillena G, Pastor I M, Ramón D J. Eur J Org Chem. 2016:612–632. doi: 10.1002/ejoc.201501197. - DOI
-
- Perna F M, Vitale P, Capriati V. Curr Opin Green Sustainable Chem. 2020;21:27–33. doi: 10.1016/j.cogsc.2019.09.004. - DOI
-
- Ramón D J, Guillena G, editors. Deep Eutectic Solvents: Synthesis, Properties, and Applications. 1st ed. Weinheim, Germany: Wiley-VCH; 2019. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
