Modulation of Ki67 and myogenic regulatory factor expression by tocotrienol-rich fraction ameliorates myogenic program of senescent human myoblasts
- PMID: 34025846
- PMCID: PMC8130490
- DOI: 10.5114/aoms.2019.85449
Modulation of Ki67 and myogenic regulatory factor expression by tocotrienol-rich fraction ameliorates myogenic program of senescent human myoblasts
Abstract
Introduction: Replicative senescence results in dysregulation of cell proliferation and differentiation, which plays a role in the regenerative defects observed during age-related muscle atrophy. Vitamin E is a well-known antioxidant, which potentially ameliorates a wide range of age-related manifestations. The aim of this study was to determine the effects of tocotrienol-rich fraction (TRF) in modulating the expression of proliferation- and differentiation-associated proteins in senescent human myoblasts during the differentiation phase.
Material and methods: Human skeletal muscle myoblasts were cultured until senescence. Young and senescent cells were treated with TRF for 24 h before and after differentiation induction, followed by evaluation of cellular morphology and efficiency of differentiation. Expression of cell proliferation marker Ki67 protein and myogenic regulatory factors MyoD and myogenin were determined.
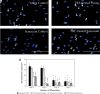
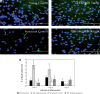
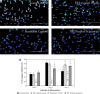
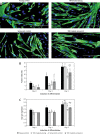
Results: Our findings showed that treatment with TRF significantly improved the morphology of senescent myoblasts. Promotion of differentiation was observed in young and senescent myoblasts with TRF treatment as shown by the increased fusion index and larger size of myotubes. Increased Ki67 and myogenin expression with TRF treatment was also observed in senescent myoblasts, suggesting amelioration of the myogenic program by TRF during replicative senescence.
Conclusions: TRF modulates the expression of regulatory factors related to proliferation and differentiation in senescent human myoblasts and could be beneficial for ameliorating the regenerative defects during aging.
Keywords: differentiation; myoblasts; myotubes; replicative senescence; tocotrienols.
Copyright: © 2019 Termedia & Banach.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Tocotrienol-Rich Fraction Is Superior to Tocopherol in Promoting Myogenic Differentiation in the Prevention of Replicative Senescence of Myoblasts.PLoS One. 2016 Feb 17;11(2):e0149265. doi: 10.1371/journal.pone.0149265. eCollection 2016. PLoS One. 2016. PMID: 26885980 Free PMC article.
-
Targeting myomiRs by tocotrienol-rich fraction to promote myoblast differentiation.Genes Nutr. 2018 Nov 29;13:31. doi: 10.1186/s12263-018-0618-2. eCollection 2018. Genes Nutr. 2018. PMID: 30519366 Free PMC article.
-
Tocotrienol-Rich Fraction Ameliorates Antioxidant Defense Mechanisms and Improves Replicative Senescence-Associated Oxidative Stress in Human Myoblasts.Oxid Med Cell Longev. 2017;2017:3868305. doi: 10.1155/2017/3868305. Epub 2017 Jan 24. Oxid Med Cell Longev. 2017. PMID: 28243354 Free PMC article.
-
Growth hormone and the insulin-like growth factor system in myogenesis.Endocr Rev. 1996 Oct;17(5):481-517. doi: 10.1210/edrv-17-5-481. Endocr Rev. 1996. PMID: 8897022 Review.
-
[Interactions of proliferation and differentiation signaling pathways in myogenesis].Postepy Hig Med Dosw (Online). 2014 May 8;68:516-26. doi: 10.5604/17322693.1101617. Postepy Hig Med Dosw (Online). 2014. PMID: 24864103 Review. Polish.
Cited by
-
Hsa_circ_0076931 suppresses malignant biological properties, down-regulates miR-6760-3p through direct binding, and up-regulates CCBE1 in glioma.Biosci Rep. 2022 Jan 28;42(1):BSR20211895. doi: 10.1042/BSR20211895. Biosci Rep. 2022. PMID: 34931668 Free PMC article.
-
Molecular Mechanism of Tocotrienol-Mediated Anticancer Properties: A Systematic Review of the Involvement of Endoplasmic Reticulum Stress and Unfolded Protein Response.Nutrients. 2023 Apr 12;15(8):1854. doi: 10.3390/nu15081854. Nutrients. 2023. PMID: 37111076 Free PMC article.
References
-
- Carosio S, Berardinelli MG, Aucello M, Musaro A. Impact of ageing on muscle cell regeneration. Ageing Res Rev. 2011;10:35–42. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
