Dynamics of antibodies to SARS-CoV-2 in convalescent plasma donors
- PMID: 34026115
- PMCID: PMC8126762
- DOI: 10.1002/cti2.1285
Dynamics of antibodies to SARS-CoV-2 in convalescent plasma donors
Abstract
Objectives: Characterisation of the human antibody response to SARS-CoV-2 infection is vital for serosurveillance purposes and for treatment options such as transfusion with convalescent plasma or immunoglobulin products derived from convalescent plasma. In this study, we longitudinally and quantitatively analysed antibody responses in RT-PCR-positive SARS-CoV-2 convalescent adults during the first 250 days after onset of symptoms.
Methods: We measured antibody responses to the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein and the nucleocapsid protein in 844 longitudinal samples from 151 RT-PCR-positive SARS-CoV-2 convalescent adults. With a median of 5 (range 2-18) samples per individual, this allowed quantitative analysis of individual longitudinal antibody profiles. Kinetic profiles were analysed by mixed-effects modelling.
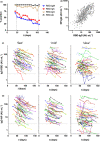
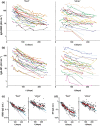
Results: All donors were seropositive at the first sampling moment, and only one donor seroreverted during follow-up analysis. Anti-RBD IgG and anti-nucleocapsid IgG levels declined with median half-lives of 62 and 59 days, respectively, 2-5 months after symptom onset, and several-fold variation in half-lives of individuals was observed. The rate of decline of antibody levels diminished during extended follow-up, which points towards long-term immunological memory. The magnitude of the anti-RBD IgG response correlated well with neutralisation capacity measured in a classic plaque reduction assay and in an in-house developed competitive assay.
Conclusion: The result of this study gives valuable insight into the long-term longitudinal response of antibodies to SARS-CoV-2.
Keywords: ACE2‐competitive ELISA; COVID‐19; antibodies; longitudinal; neutralisation.
© 2021 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Casadevall A, Joyner MJ, Pirofski L‐A. A randomized trial of convalescent plasma for COVID‐19—potentially hopeful signals. JAMA 2020; 324: 455–457. - PubMed
-
- Chai KL, Valk SJ, Piechotta V et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database Syst Rev 2020; 10: CD013600. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
