CD38-Targeted Theranostics of Lymphoma with 89Zr/177Lu-Labeled Daratumumab
- PMID: 34026426
- PMCID: PMC8132161
- DOI: 10.1002/advs.202001879
CD38-Targeted Theranostics of Lymphoma with 89Zr/177Lu-Labeled Daratumumab
Abstract
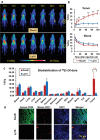
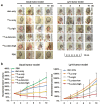
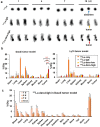
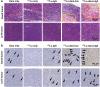
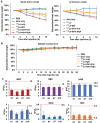
Lymphoma is a heterogeneous disease with varying clinical manifestations and outcomes. Many subtypes of lymphoma, such as Burkitt's lymphoma and diffuse large B cell lymphoma, are highly aggressive with dismal prognosis even after conventional chemotherapy and radiotherapy. As such, exploring specific biomarkers for lymphoma is of high clinical significance. Herein, a potential marker, CD38, is investigated for differentiating lymphoma. A CD38-targeting monoclonal antibody (mAb, daratumumab) is then radiolabeled with Zr-89 and Lu-177 for theranostic applications. As the diagnostic component, the Zr-89-labeled mAb is highly specific in delineating CD38-positive lymphoma via positron emission tomography (PET) imaging, while the Lu-177-labeled mAb serves well as the therapeutic component to suppress tumor growth after a one-time administration. These results strongly suggest that CD38 is a lymphoma-specific marker and prove that 89Zr/177Lu-labeled daratumumab facilitates immunoPET imaging and radioimmunotherapy of lymphoma in preclinical models. Further clinical evaluation and translation of this CD38-targeted theranostics may be of significant help in lymphoma patient stratification and management.
Keywords: CD38; Lu‐177; daratumumab; lymphoma; positron emission tomography (PET); radioimmunotherapy; theranostics.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
Weibo Cai is scientific advisor, stockholder, and grantee of Focus‐X Therapeutics, Inc. The other authors declare no conflict of interest.
Figures


References
-
- Siegel R. L., Miller K. D., Jemal A., Ca‐Cancer J. Clin. 2018, 68, 7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
