Gut Microbiota: Influence on Carcinogenesis and Modulation Strategies by Drug Delivery Systems to Improve Cancer Therapy
- PMID: 34026439
- PMCID: PMC8132165
- DOI: 10.1002/advs.202003542
Gut Microbiota: Influence on Carcinogenesis and Modulation Strategies by Drug Delivery Systems to Improve Cancer Therapy
Abstract
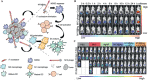
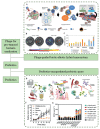
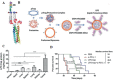
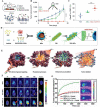
Gut microbiota have close interactions with the host. It can affect cancer progression and the outcomes of cancer therapy, including chemotherapy, immunotherapy, and radiotherapy. Therefore, approaches toward the modulation of gut microbiota will enhance cancer prevention and treatment. Modern drug delivery systems (DDS) are emerging as rational and promising tools for microbiota intervention. These delivery systems have compensated for the obstacles associated with traditional treatments. In this review, the essential roles of gut microbiota in carcinogenesis, cancer progression, and various cancer therapies are first introduced. Next, advances in DDS that are aimed at enhancing the efficacy of cancer therapy by modulating or engineering gut microbiota are highlighted. Finally, the challenges and opportunities associated with the application of DDS targeting gut microbiota for cancer prevention and treatment are briefly discussed.
Keywords: cancer therapy; carcinogenesis; drug delivery system; gut microbiota; modulation.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Intervention on gut microbiota may change the strategy for management of colorectal cancer.J Gastroenterol Hepatol. 2021 Jun;36(6):1508-1517. doi: 10.1111/jgh.15369. Epub 2021 Jan 5. J Gastroenterol Hepatol. 2021. PMID: 33295040 Review.
-
Microbiota transplantation: Targeting cancer treatment.Cancer Lett. 2019 Jun 28;452:144-151. doi: 10.1016/j.canlet.2019.03.010. Epub 2019 Mar 21. Cancer Lett. 2019. PMID: 30905818 Review.
-
The gut microbiome and cancer: from tumorigenesis to therapy.Nat Metab. 2025 May;7(5):895-917. doi: 10.1038/s42255-025-01287-w. Epub 2025 May 6. Nat Metab. 2025. PMID: 40329009 Review.
-
Novel strategies for modulating the gut microbiome for cancer therapy.Adv Drug Deliv Rev. 2024 Jul;210:115332. doi: 10.1016/j.addr.2024.115332. Epub 2024 May 15. Adv Drug Deliv Rev. 2024. PMID: 38759702 Free PMC article. Review.
-
Emerging role of microbiota in immunomodulation and cancer immunotherapy.Semin Cancer Biol. 2021 May;70:37-52. doi: 10.1016/j.semcancer.2020.06.008. Epub 2020 Jun 21. Semin Cancer Biol. 2021. PMID: 32580024 Review.
Cited by
-
Microbial Therapy and Breast Cancer Management: Exploring Mechanisms, Clinical Efficacy, and Integration within the One Health Approach.Int J Mol Sci. 2024 Jan 16;25(2):1110. doi: 10.3390/ijms25021110. Int J Mol Sci. 2024. PMID: 38256183 Free PMC article. Review.
-
Effects of Docetaxel Injection and Docetaxel Micelles on the Intestinal Barrier and Intestinal Microbiota.Adv Sci (Weinh). 2021 Dec;8(24):e2102952. doi: 10.1002/advs.202102952. Epub 2021 Oct 28. Adv Sci (Weinh). 2021. PMID: 34713626 Free PMC article.
-
Advances in Hydrogel-Based Delivery of RNA Drugs for Antitumor Therapy.Gels. 2025 Aug 11;11(8):633. doi: 10.3390/gels11080633. Gels. 2025. PMID: 40868764 Free PMC article. Review.
-
Intracellular hydrogelation of macrophage conjugated probiotics for hitchhiking delivery and combined treatment of colitis.Mater Today Bio. 2023 May 19;20:100679. doi: 10.1016/j.mtbio.2023.100679. eCollection 2023 Jun. Mater Today Bio. 2023. PMID: 37273799 Free PMC article.
-
Yishen Qingli Heluo Granule Ameliorates Renal Dysfunction in 5/6 Nephrectomized Rats by Targeting Gut Microbiota and Intestinal Barrier Integrity.Front Pharmacol. 2022 Jun 22;13:858881. doi: 10.3389/fphar.2022.858881. eCollection 2022. Front Pharmacol. 2022. PMID: 35814258 Free PMC article.
References
-
- a) Cho I., Blaser M. J., Nat. Rev. Genet. 2012, 13, 260; - PMC - PubMed
- b) Costello E. K., Lauber C. L., Hamady M., Fierer N., Gordon J. I., Knight R., Science 2009, 326, 1694; - PMC - PubMed
- c) Gilbert J. A., Blaser M. J., Caporaso J. G., Jansson J. K., Lynch S. V., Knight R., Nat. Med. 2018, 24, 392. - PMC - PubMed
-
- Roy S., Trinchieri G., Nat. Rev. Cancer 2017, 17, 271. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
