Engineered Matrices Enable the Culture of Human Patient-Derived Intestinal Organoids
- PMID: 34026461
- PMCID: PMC8132048
- DOI: 10.1002/advs.202004705
Engineered Matrices Enable the Culture of Human Patient-Derived Intestinal Organoids
Abstract
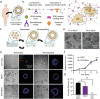
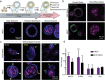
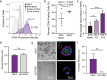
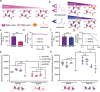
Human intestinal organoids from primary human tissues have the potential to revolutionize personalized medicine and preclinical gastrointestinal disease models. A tunable, fully defined, designer matrix, termed hyaluronan elastin-like protein (HELP) is reported, which enables the formation, differentiation, and passaging of adult primary tissue-derived, epithelial-only intestinal organoids. HELP enables the encapsulation of dissociated patient-derived cells, which then undergo proliferation and formation of enteroids, spherical structures with polarized internal lumens. After 12 rounds of passaging, enteroid growth in HELP materials is found to be statistically similar to that in animal-derived matrices. HELP materials also support the differentiation of human enteroids into mature intestinal cell subtypes. HELP matrices allow stiffness, stress relaxation rate, and integrin-ligand concentration to be independently and quantitatively specified, enabling fundamental studies of organoid-matrix interactions and potential patient-specific optimization. Organoid formation in HELP materials is most robust in gels with stiffer moduli (G' ≈ 1 kPa), slower stress relaxation rate (t1/2 ≈ 18 h), and higher integrin ligand concentration (0.5 × 10-3-1 × 10-3 m RGD peptide). This material provides a promising in vitro model for further understanding intestinal development and disease in humans and a reproducible, biodegradable, minimal matrix with no animal-derived products or synthetic polyethylene glycol for potential clinical translation.
Keywords: 3D cell culture; adult stem cells; engineered biomaterial; extracellular matrix; intestinal organoid.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bar‐Ephraim Y. E., Kretzschmar K., Clevers H., Nat. Rev. Immunol. 2019, 20, 279. - PubMed
-
- Huch M., Knoblich J. A., Lutolf M. P., Martinez‐Arias A., Development 2017, 144, 938. - PubMed
-
- Rossi G., Manfrin A., Lutolf M. P., Nat. Rev. Genet. 2018, 19, 671. - PubMed
-
- Sato T., Vries R. G., Snippert H. J., van de Wetering M., Barker N., Stange D. E., van Es J. H., Abo A., Kujala P., Peters P. J., Clevers H., Nature 2009, 459, 262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
