A randomized controlled study in healthy participants to explore the exposure continuum when smokers switch to a tobacco heating product or an E-cigarette relative to cessation
- PMID: 34026564
- PMCID: PMC8131274
- DOI: 10.1016/j.toxrep.2021.05.003
A randomized controlled study in healthy participants to explore the exposure continuum when smokers switch to a tobacco heating product or an E-cigarette relative to cessation
Abstract
Background: Cigarette smoking is associated with a number of diseases, such as cancer and cardiovascular diseases. Recently, there has been an increase in the use of electronic cigarettes (ECs) and tobacco-heating products (THPs) as an alternative to cigarettes, which may reduce the health burden associated with smoking. However, an exposure continuum when smokers switch to ECs or THPs compared to complete smoking cessation is not well established.
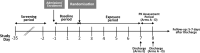
Methods: 148 healthy smokers were randomized to either continue smoking cigarettes, switch to using the glo THP or a prototype EC, or completely quit any nicotine or tobacco product use for 5 days, after a 2-day baseline period. During this study breath and 24-h urine samples were collected for Biomarker of Exposure (BoE) analysis.
Results: After a 5-day switching period BoE levels showed a substantial significant decrease in levels from baseline in the groups using the glo THP, the prototype EC, and having quit all nicotine and tobacco use. On an exposure continuum, smokers who completely quit nicotine had the lowest levels of assessed BoEs, followed by those who switched to the EC and then those who switched to glo THP use. Participants who continued to smoke had the highest levels of BoEs.
Conclusions: THP or EC use over a 5-day period resulted in significant reductions in exposure to smoke toxicants, in some cases to levels similar to those for nicotine cessation. These results show that on an exposure continuum, nicotine cessation gives the greatest reduction in exposure to tobacco smoke toxicants, closely followed by the EC and the glo THP. These significant reductions in exposure to toxicants suggest that the glo THP and EC have the potential to be Reduced Risk Products.
Study registration: ISRCTN80651909.
Keywords: Biomarker of exposure; Harm reduction; Smoking; Tobacco heating product; e-Cigarette.
© 2021 British American Tobacco (Investments) Ltd.
Conflict of interest statement
All authors except CJP are current employees of British American Tobacco (Investments) Limited. CJP is a consultant contracted by British American Tobacco (Investments) Limited.
Figures


Similar articles
-
Changes in Biomarkers of Exposure on Switching From a Conventional Cigarette to the glo Tobacco Heating Product: A Randomized, Controlled Ambulatory Study.Nicotine Tob Res. 2021 Feb 16;23(3):584-591. doi: 10.1093/ntr/ntaa135. Nicotine Tob Res. 2021. PMID: 32776101 Free PMC article. Clinical Trial.
-
Changes in biomarkers of exposure and biomarkers of potential harm after 360 days in smokers who either continue to smoke, switch to a tobacco heating product or quit smoking.Intern Emerg Med. 2022 Oct;17(7):2017-2030. doi: 10.1007/s11739-022-03062-1. Epub 2022 Aug 28. Intern Emerg Med. 2022. PMID: 36036342 Free PMC article.
-
Changes in Biomarkers of Exposure on Switching From a Conventional Cigarette to Tobacco Heating Products: A Randomized, Controlled Study in Healthy Japanese Subjects.Nicotine Tob Res. 2019 Aug 19;21(9):1220-1227. doi: 10.1093/ntr/nty104. Nicotine Tob Res. 2019. PMID: 29912406 Free PMC article. Clinical Trial.
-
Evaluation of behavioural, chemical, toxicological and clinical studies of a tobacco heated product glo™ and the potential for bridging from a foundational dataset to new product iterations.Toxicol Rep. 2022 Jun 25;9:1426-1442. doi: 10.1016/j.toxrep.2022.06.014. eCollection 2022. Toxicol Rep. 2022. PMID: 36561950 Free PMC article. Review.
-
[Smoking reduction and temporary abstinence: new approaches for smoking cessation].J Mal Vasc. 2003 Dec;28(5):293-300. J Mal Vasc. 2003. PMID: 14978435 Review. French.
Cited by
-
Comparative toxicological assessment of cigarettes and new category products via an in vitro multiplex proteomics platform.Toxicol Rep. 2024 May 6;12:492-501. doi: 10.1016/j.toxrep.2024.04.006. eCollection 2024 Jun. Toxicol Rep. 2024. PMID: 38774478 Free PMC article.
-
A Scoping Review of Behavioural Studies on Heated Tobacco Products.Cureus. 2024 Jul 30;16(7):e65773. doi: 10.7759/cureus.65773. eCollection 2024 Jul. Cureus. 2024. PMID: 39211653 Free PMC article.
-
A randomised, crossover, clinical study to assess nicotine pharmacokinetics and subjective effects of the BIDI® stick ENDS compared with combustible cigarettes and a comparator ENDS in adult smokers.Harm Reduct J. 2022 Jun 2;19(1):57. doi: 10.1186/s12954-022-00638-0. Harm Reduct J. 2022. PMID: 35655314 Free PMC article. Clinical Trial.
-
Exposure to Tobacco-Specific Nitrosamines Among People Who Vape, Smoke, or do Neither: A Systematic Review and Meta-Analysis.Nicotine Tob Res. 2024 Feb 22;26(3):257-269. doi: 10.1093/ntr/ntad156. Nicotine Tob Res. 2024. PMID: 37619211 Free PMC article.
-
Designing Studies to Inform Tobacco Harm Reduction: Learnings From an Oral Nicotine Pouch Actual Use Pilot Study.JMIR Form Res. 2022 Aug 19;6(8):e37573. doi: 10.2196/37573. JMIR Form Res. 2022. PMID: 35984682 Free PMC article.
References
-
- Perfetti T., Rodgman A. The complexity of tobacco and tobacco smoke. Beitr. Tabaforsch. 2011;24(5):215–232. doi: 10.2478/cttr-2013-0902. - DOI
-
- US Department of Health and Human Services . Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta: 2014. The Health Consequences of Smoking: 50 Years of Progress: a Report of the Surgeon General.
LinkOut - more resources
Full Text Sources
Other Literature Sources

