Case Report: Prolonged Survival Following EGFRvIII CAR T Cell Treatment for Recurrent Glioblastoma
- PMID: 34026647
- PMCID: PMC8138201
- DOI: 10.3389/fonc.2021.669071
Case Report: Prolonged Survival Following EGFRvIII CAR T Cell Treatment for Recurrent Glioblastoma
Abstract
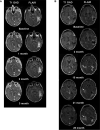
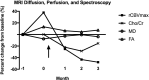
Autologous chimeric antigen receptor (CAR) T cells targeted to epidermal growth factor receptor variant III (CAR T-EGFRvIII) have been developed and administered experimentally to treat patients with IDH1 wildtype recurrent glioblastoma (rGBM) (NCT02209376). We report the case of a 59-year-old patient who received a single peripheral infusion of CAR T-EGFRvIII cells and survived 36 months after disease recurrence, exceeding expected survival for recurrent glioblastoma. Post-infusion histopathologic analysis of tissue obtained during a second stage surgical resection revealed immunosuppressive adaptive changes in the tumor tissue as well as reduced EGFRvIII expression. Serial brain imaging demonstrated a significant reduction in relative cerebral blood volume (rCBV), a measure strongly associated with tumor proliferative activity, at early time points following CAR T treatment. Notably, CAR T-EGFRvIII cells persisted in her peripheral circulation during 29 months of follow-up, the longest period of CAR T persistence reported in GBM trials to date. These findings in a long-term survivor show that peripherally administered CAR T-EGFRvIII cells can persist for years in the circulation and suggest that this cell therapy approach could be optimized to achieve broader efficacy in recurrent GBM patients.
Keywords: CAR (chimeric antigen receptor); CAR T cell therapy; EGFRvIII; glioblastoma; perfusion imaging; recurrent glioblastoma (rGBM).
Copyright © 2021 Durgin, Henderson, Nasrallah, Mohan, Wang, Lacey, Melenhorst, Desai, Lee, Maus, June, Brem, O’Connor, Binder and O’Rourke.
Conflict of interest statement
DO’R and ZB are inventors on patents related to CAR T cells that have been filed by the University of Pennsylvania. JJM consults with or serves on the board of directors of several companies developing CAR T technology. JM and SL are inventors of intellectual property related to CAR T cells that is licensed by the University of Pennsylvania to Novartis. CHJ reports receiving grants from Tmunity Therapeutics and holds founders stock in Tmunity Therapeutics and DeCART Therapeutics. CHJ also receives personal income from BluesphereBio, Cabaletta, Carisma, Cellares, Celldex Therapeutics, Viracta Therapeutics, Ziopharm and WIRB-Copernicus Group as well as royalties from Novartis. MCM is an inventor on patent applications related to CAR technology and has received licensing royalties from Novartis corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJ, et al. A Single Dose of Peripherally Infused EGFRvIII-directed Car T Cells Mediates Antigen Loss and Induces Adaptive Resistance in Patients With Recurrent Glioblastoma. Sci Trans Med (2017) 9(399):eaaa0984. 10.1126/scitranslmed.aaa0984 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

