The High-Spin Heme b L Mutant Exposes Dominant Reaction Leading to the Formation of the Semiquinone Spin-Coupled to the [2Fe-2S]+ Cluster at the Qo Site of Rhodobacter capsulatus Cytochrome bc 1
- PMID: 34026724
- PMCID: PMC8138165
- DOI: 10.3389/fchem.2021.658877
The High-Spin Heme b L Mutant Exposes Dominant Reaction Leading to the Formation of the Semiquinone Spin-Coupled to the [2Fe-2S]+ Cluster at the Qo Site of Rhodobacter capsulatus Cytochrome bc 1
Abstract
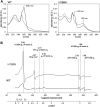
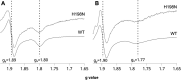
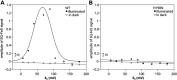
Cytochrome bc 1 (mitochondrial complex III) catalyzes electron transfer from quinols to cytochrome c and couples this reaction with proton translocation across lipid membrane; thus, it contributes to the generation of protonmotive force used for the synthesis of ATP. The energetic efficiency of the enzyme relies on a bifurcation reaction taking place at the Qo site which upon oxidation of ubiquinol directs one electron to the Rieske 2Fe2S cluster and the other to heme b L. The molecular mechanism of this reaction remains unclear. A semiquinone spin-coupled to the reduced 2Fe2S cluster (SQo-2Fe2S) was identified as a state associated with the operation of the Qo site. To get insights into the mechanism of the formation of this state, we first constructed a mutant in which one of the histidine ligands of the iron ion of heme b L Rhodobacter capsulatus cytochrome bc 1 was replaced by asparagine (H198N). This converted the low-spin, low-potential heme into the high-spin, high-potential species which is unable to support enzymatic turnover. We performed a comparative analysis of redox titrations of antimycin-supplemented bacterial photosynthetic membranes containing native enzyme and the mutant. The titrations revealed that H198N failed to generate detectable amounts of SQo-2Fe2S under neither equilibrium (in dark) nor nonequilibrium (in light), whereas the native enzyme generated clearly detectable SQo-2Fe2S in light. This provided further support for the mechanism in which the back electron transfer from heme b L to a ubiquinone bound at the Qo site is mainly responsible for the formation of semiquinone trapped in the SQo-2Fe2S state in R. capusulatus cytochrome bc 1.
Keywords: cytochrome bc1 (complex III); electron paramagnetic resonance; electron transfer; quinol oxidation; semiquinone.
Copyright © 2021 Sarewicz, Pintscher, Bujnowicz, Wolska and Artur Osyczka.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Al-Attar S., de Vries S. (2013). Energy Transduction by Respiratory Metallo-Enzymes: From Molecular Mechanism to Cell Physiology. Coord. Chem. Rev. 257, 64–80. 10.1016/j.ccr.2012.05.022 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

