Droplet-Based Microfluidic High Throughput Screening of Corynebacterium glutamicum for Efficient Heterologous Protein Production and Secretion
- PMID: 34026744
- PMCID: PMC8137953
- DOI: 10.3389/fbioe.2021.668513
Droplet-Based Microfluidic High Throughput Screening of Corynebacterium glutamicum for Efficient Heterologous Protein Production and Secretion
Abstract
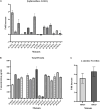
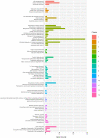
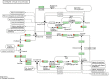
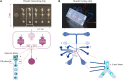
With emerging interests in heterologous production of proteins such as antibodies, growth factors, nanobodies, high-quality protein food ingredients, etc. the demand for efficient production hosts increases. Corynebacterium glutamicum is an attractive industrial host with great secretion capacity to produce therapeutics. It lacks extracellular protease and endotoxin activities and easily achieves high cell density. Therefore, this study focuses on improving protein production and secretion in C. glutamicum with the use of droplet-based microfluidic (DBM) high throughput screening. A library of C. glutamicum secreting β-glucosidase was generated using chemical mutagenesis coupled with DBM screening of 200,000 mutants in just 20 min. Among 100 recovered mutants, 16 mutants exhibited enhanced enzyme secretion capacity, 13 of which had unique mutation profiles. Whole-genome analysis showed that approximately 50-150 SNVs had occurred on the chromosome per mutant. Functional enrichment analysis of genes with non-synonymous mutations showed overrepresentation of genes involved in protein synthesis and secretion relevant biological processes, such as DNA and ribosome RNA synthesis, protein secretion and energy turnover. Two mutants JCMT1 and JCMT8 exhibited the highest secretion with a six and a fivefold increase in the β-glucosidase activity in the supernatant, respectively, relative to the reference strain JC0190. After plasmid curing, a new plasmid with the gene encoding α-amylase was cloned into these two mutants. The new strains SB024 and SB025 also exhibited a five and a sixfold increase in α-amylase activity in the supernatant, respectively, relative to the reference strain SB023. The results demonstrate how DBM screening can serve as a powerful development tool to improve cell factories for the production and secretion of heterologous proteins.
Keywords: Corynebacterium glutamicum; droplet-based microfluidics; heterologous protein production; high throughput screening; α-amylase; β-glucosidase.
Copyright © 2021 Balasubramanian, Chen, Wigneswaran, Bang-Berthelsen and Jensen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
A secretion biosensor for monitoring Sec-dependent protein export in Corynebacterium glutamicum.Microb Cell Fact. 2020 Jan 21;19(1):11. doi: 10.1186/s12934-019-1273-z. Microb Cell Fact. 2020. PMID: 31964372 Free PMC article.
-
Enhancement of heterologous protein production in Corynebacterium glutamicum via atmospheric and room temperature plasma mutagenesis and high-throughput screening.J Biotechnol. 2021 Sep 20;339:22-31. doi: 10.1016/j.jbiotec.2021.07.010. Epub 2021 Jul 24. J Biotechnol. 2021. PMID: 34311028
-
[Development and application of a droplet-based microfluidic high-throughput screening of Pichia pastoris].Sheng Wu Gong Cheng Xue Bao. 2019 Jul 25;35(7):1317-1325. doi: 10.13345/j.cjb.190058. Sheng Wu Gong Cheng Xue Bao. 2019. PMID: 31328488 Chinese.
-
Protein secretion in Corynebacterium glutamicum.Crit Rev Biotechnol. 2017 Jun;37(4):541-551. doi: 10.1080/07388551.2016.1206059. Epub 2016 Oct 13. Crit Rev Biotechnol. 2017. PMID: 27737570 Review.
-
Expression of recombinant protein using Corynebacterium Glutamicum: progress, challenges and applications.Crit Rev Biotechnol. 2016 Aug;36(4):652-64. doi: 10.3109/07388551.2015.1004519. Epub 2015 Feb 25. Crit Rev Biotechnol. 2016. PMID: 25714007 Review.
Cited by
-
Exploring the secretome of Corynebacterium glutamicum ATCC 13032.Front Bioeng Biotechnol. 2024 Feb 13;12:1348184. doi: 10.3389/fbioe.2024.1348184. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38415189 Free PMC article.
-
Protective Cultures in Food Products: From Science to Market.Foods. 2023 Apr 5;12(7):1541. doi: 10.3390/foods12071541. Foods. 2023. PMID: 37048362 Free PMC article. Review.
-
The use of droplet-based microfluidic technologies for accelerated selection of Yarrowia lipolytica and Phaffia rhodozyma yeast mutants.Biol Methods Protoc. 2024 Jul 10;9(1):bpae049. doi: 10.1093/biomethods/bpae049. eCollection 2024. Biol Methods Protoc. 2024. PMID: 39114747 Free PMC article. Review.
-
Comprehensive Analysis of Multi-Omics Data on RNA Polymerase as an Adverse Factor in Head and Neck Squamous Cell Carcinoma.J Inflamm Res. 2025 Mar 1;18:3067-3091. doi: 10.2147/JIR.S496748. eCollection 2025. J Inflamm Res. 2025. PMID: 40051449 Free PMC article.
-
Single-Cell Technologies to Study Phenotypic Heterogeneity and Bacterial Persisters.Microorganisms. 2021 Nov 1;9(11):2277. doi: 10.3390/microorganisms9112277. Microorganisms. 2021. PMID: 34835403 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

