All Hands-On Deck and All Decks on Hand: Surmounting Supply Chain Limitations During the COVID-19 Pandemic
- PMID: 34027053
- PMCID: PMC8120534
- DOI: 10.1177/23742895211011928
All Hands-On Deck and All Decks on Hand: Surmounting Supply Chain Limitations During the COVID-19 Pandemic
Abstract
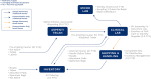
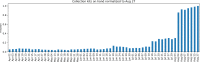
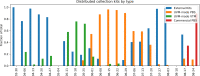
Testing during the COVID-19 pandemic has been crucial to public health surveillance and clinical care. Supply chain constraints-spanning limitations in testing kits, reagents, pipet tips, and swabs availability-have challenged the ability to scale COVID-19 testing. During the early months, sample collection kits shortages constrained planned testing expansions. In response, the University of Vermont Medical Center, University of Vermont College of Medicine, Vermont Department of Health Laboratory, Aspenti Health, and providers across Vermont including 16 area hospitals partnered to surmount these barriers. The primary objectives were to increase supply availability and manage utilization. Within the first month of Vermont's stay-at-home order, the University of Vermont Medical Center laboratory partnered with College of Medicine to create in-house collection kits, producing 5000 per week. University of Vermont Medical Center reassigned 4 phlebotomists, laboratory educators, and other laboratory staff, who had reduced workloads, to participate (requiring a total of 5.3-7.6 full-time equivalent (FTE) during the period of study). By August, automation at a local commercial laboratory produced 22,000 vials of media in one week (reducing the required personnel by 1.2 FTE). A multisite, cross-institutional approach was used to manage specimen collection kit utilization across Vermont. Hospital laboratory directors, managers, and providers agreed to order only as needed to avoid supply stockpiles and supported operational constraints through ongoing validations and kit assembly. Throughout this pandemic, Vermont has ranked highly in number of tests per million people, demonstrating the value of local collaboration to surmount obstacles during disease outbreaks and the importance of creative allocation of resources to address statewide needs.
Keywords: COVID-19; inventory management; pandemic; specimen collection kits; supply chain.
© The Author(s) 2021.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Vanessa Clark and Lauren Risley are salaried by Aspenti Health, a laboratory focused on population health management for Substance Use Disorders. Vanessa Clark holds a small portion of stock options (<$1000 in value) at Aspenti Health. Jill S. Warrington carries a nonsalaried position as Chief Medical Officer at Aspenti Health where she holds a small portion of stock options (<$1000 in value). She also is a member of the community advisory council at Blue Cross Blue Shield of Vermont. Mark Fung serves as an ad hoc consultant for Cerus Corporation and Biocogniv. He also receives honorariums from Grifols Corporation for educational presentations.
Figures




References
-
- COVID-19 Coronavirus Pandemic. Worldometer.info. Published 2020. Updated March 3, 2021. Accessed March 3, 2021. https://www.worldometers.info/coronavirus/
-
- Coronavirus Resource Center. Johns Hopkins University. Published 2020. Updated February 26, 2021. Accessed February 26, 2021. https://coronavirus.jhu.edu/map.html/
-
- Thurston J, Markos M. Fauci: Vermont a ‘Model’ for the country on coronavirus response. New York Times. September 15, 2020.
-
- Collins K. Is your state doing enough coronavirus testing?. New York Times. January 1, 2021.
LinkOut - more resources
Full Text Sources
Other Literature Sources

