A review of biomarkers in the context of type 1 diabetes: Biological sensing for enhanced glucose control
- PMID: 34027090
- PMCID: PMC8126822
- DOI: 10.1002/btm2.10201
A review of biomarkers in the context of type 1 diabetes: Biological sensing for enhanced glucose control
Abstract
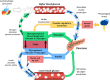
As wearable healthcare monitoring systems advance, there is immense potential for biological sensing to enhance the management of type 1 diabetes (T1D). The aim of this work is to describe the ongoing development of biomarker analytes in the context of T1D. Technological advances in transdermal biosensing offer remarkable opportunities to move from research laboratories to clinical point-of-care applications. In this review, a range of analytes, including glucose, insulin, glucagon, cortisol, lactate, epinephrine, and alcohol, as well as ketones such as beta-hydroxybutyrate, will be evaluated to determine the current status and research direction of those analytes specifically relevant to T1D management, using both in-vitro and on-body detection. Understanding state-of-the-art developments in biosensing technologies will aid in bridging the gap from bench-to-clinic T1D analyte measurement advancement.
Keywords: automated insulin delivery; biosensors; measurement; medical devices; nanobiology; type 1 diabetes.
© 2020 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
Dr. Dassau reports receiving grants from JDRF, NIH, and Helmsley Charitable Trust, personal fees from Roche and Eli Lilly, patents on artificial pancreas technology, and product support from Dexcom, Insulet, Tandem, and Roche. Dr. Dassau is currently an employee and shareholder of Eli Lilly and Company. The work presented in this manuscript was performed as part of his academic appointment and is independent of his employment with Eli Lilly and Company. Dr. Doyle reports equity, licensed IP and is a member of the Scientific Advisory Board of Mode AGC. Dr. Laffel reports grant support to her institution from NIH, JDRF, Helmsley Charitable Trust, Eli Lilly and Company, Insulet, Dexcom, and Boehringer Ingelheim; she receives consulting fees unrelated to the current report from Johnson & Johnson, Sanofi, NovoNordisk, Roche, Dexcom, Insulet, Boehringer Ingelheim, ConvaTec, Medtronic, Lifescan, Laxmi, and Insulogic. Dr. Patti reports receiving grant support, provided to her institution, from NIH, Helmsely Charitable Trust, Chan Zuckerberg Foundation, and Dexcom, patents related to hypoglycemia and pump therapy for hypoglycemia, and advisory board fees from Fractyl (unrelated to the current report). Dr. Pinsker reports grant support, provided to his institution, consulting fees, and speaker fees from Tandem Diabetes Care, grant support, provided to his institution, and advisory board fees from Medtronic, grant support, provided to his institution, and consulting fees from Eli Lilly, grant support and supplies, provided to his institution, from Insulet, and supplies, provided to his institution, from Dexcom.
Figures

References
-
- Katsarou A, Gudbjörnsdottir S, Rawshani A, et al. Type 1 diabetes mellitus. Nature Reviews Disease Primers. 2017;3(1):1‐17. - PubMed
-
- Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2020.
-
- American Diabetes Association, others . Standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Supplement 1):S1‐S212. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

