Mitochondrial transplantation therapy inhibit carbon tetrachloride-induced liver injury through scavenging free radicals and protecting hepatocytes
- PMID: 34027095
- PMCID: PMC8126821
- DOI: 10.1002/btm2.10209
Mitochondrial transplantation therapy inhibit carbon tetrachloride-induced liver injury through scavenging free radicals and protecting hepatocytes
Abstract
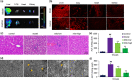
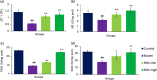
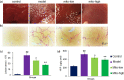
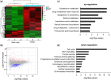
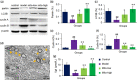
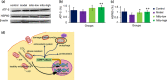
Carbon tetrachloride (CCl4)-induced liver injury is predominantly caused by free radicals, in which mitochondrial function of hepatocytes is impaired, accompanying with the production of ROS and decreased ATP energy supply in animals intoxicated with CCl4. Here we explored a novel therapeutic approach, mitochondrial transplantation therapy, for treating the liver injury. The results showed that mitochondria entered hepatocytes through macropinocytosis pathway, and thereby cell viability was recovered in a concentration-dependent manner. Mitochondrial therapy could increase ATP supply and reduce free radical damage. In liver injury model of mice, mitochondrial therapy significantly improved liver function and prevented tissue fibrogenesis. Transcriptomic data revealed that mitochondrial unfold protein response (UPRmt), a protective transcriptional response of mitochondria-to-nuclear retrograde signaling, would be triggered after mitochondrial administration. Then the anti-oxidant genes were up-regulated to scavenge free radicals. The mitochondrial function was rehabilitated through the transcriptional activation of respiratory chain enzyme and mitophage-associated genes. The protective response re-balanced the cellular homeostasis, and eventually enhanced stress resistance that is linked to cell survival. The efficacy of mitochondrial transplantation therapy in the animals would suggest a novel approach for treating liver injury caused by toxins.
Keywords: UPRmt; energy supply; free radical; mitochondrial therapy.
© 2020 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of The American Institute of Chemical Engineers.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures



References
-
- Kim YO, Popov Y, Schuppan D. Optimized mouse models for liver fibrosis. Method Mol Biol. 2017;1559:279‐296. - PubMed
LinkOut - more resources
Full Text Sources

