Cell therapies in the clinic
- PMID: 34027097
- PMCID: PMC8126820
- DOI: 10.1002/btm2.10214
Cell therapies in the clinic
Abstract
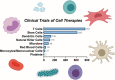
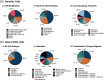
Cell therapies have emerged as a promising therapeutic modality with the potential to treat and even cure a diverse array of diseases. Cell therapies offer unique clinical and therapeutic advantages over conventional small molecules and the growing number of biologics. Particularly, living cells can simultaneously and dynamically perform complex biological functions in ways that conventional drugs cannot; cell therapies have expanded the spectrum of available therapeutic options to include key cellular functions and processes. As such, cell therapies are currently one of the most investigated therapeutic modalities in both preclinical and clinical settings, with many products having been approved and many more under active clinical investigation. Here, we highlight the diversity and key advantages of cell therapies and discuss their current clinical advances. In particular, we review 28 globally approved cell therapy products and their clinical use. We also analyze >1700 current active clinical trials of cell therapies, with an emphasis on discussing their therapeutic applications. Finally, we critically discuss the major biological, manufacturing, and regulatory challenges associated with the clinical translation of cell therapies.
Keywords: T cell; blood cell; cell; cell therapy; clinical translation; clinical trials; microbes; stem cell.
© 2021 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

