Zn-Mg and Zn-Cu alloys for stenting applications: From nanoscale mechanical characterization to in vitro degradation and biocompatibility
- PMID: 34027233
- PMCID: PMC8121665
- DOI: 10.1016/j.bioactmat.2021.04.015
Zn-Mg and Zn-Cu alloys for stenting applications: From nanoscale mechanical characterization to in vitro degradation and biocompatibility
Abstract
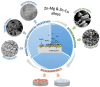
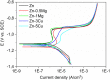
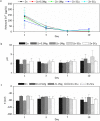
In the recent decades, zinc (Zn) and its alloys have been drawing attention as promising candidates for bioresorbable cardiovascular stents due to its degradation rate more suitable than magnesium (Mg) and iron (Fe) alloys. However, its mechanical properties need to be improved in order to meet the criteria for vascular stents. This work investigates the mechanical properties, biodegradability and biocompatibility of Zn-Mg and Zn-Cu alloys in order to determine a proper alloy composition for optimal stent performance. Nanoindentation measurements are performed to characterize the mechanical properties at the nanoscale as a function of the Zn microstructure variations induced by alloying. The biodegradation mechanisms are discussed and correlated to microstructure, mechanical performance and bacterial/cell response. Addition of Mg or Cu alloying elements refined the microstructure of Zn and enhanced yield strength (YS) and ultimate tensile strength (UTS) proportional to the volume fraction of secondary phases. Zn-1Mg showed the higher YS and UTS and better performance in terms of degradation stability in Hanks' solution. Zn-Cu alloys presented an antibacterial effect for S. aureus controlled by diffusion mechanisms and by contact. Biocompatibility was dependent on the degradation rate and the nature of the corrosion products.
Keywords: Biocompatibility; Bioresorbable metals; Galvanic corrosion; Nanoindentation; Zinc alloys.
© 2021 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





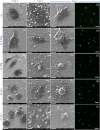
 -2h,
-2h,  -4h,
-4h,  -8h,
-8h,  -26h,
-26h,  -2d,
-2d,  -3d, and
-3d, and  -7d.
-7d.


Similar articles
-
The enhancement of mechanical properties and uniform degradation of electrodeposited Fe-Zn alloys by multilayered design for biodegradable stent applications.Acta Biomater. 2023 Apr 15;161:309-323. doi: 10.1016/j.actbio.2023.02.029. Epub 2023 Feb 27. Acta Biomater. 2023. PMID: 36858165
-
Biodegradable Zn-Dy binary alloys with high strength, ductility, cytocompatibility, and antibacterial ability for bone-implant applications.Acta Biomater. 2023 Jan 1;155:684-702. doi: 10.1016/j.actbio.2022.10.053. Epub 2022 Nov 1. Acta Biomater. 2023. PMID: 36328128
-
Impact of gadolinium on mechanical properties, corrosion resistance, and biocompatibility of Zn-1Mg-xGd alloys for biodegradable bone-implant applications.Acta Biomater. 2022 Apr 1;142:361-373. doi: 10.1016/j.actbio.2022.02.015. Epub 2022 Feb 18. Acta Biomater. 2022. PMID: 35189378
-
Zinc-based alloys for degradable vascular stent applications.Acta Biomater. 2018 Apr 15;71:1-23. doi: 10.1016/j.actbio.2018.03.005. Epub 2018 Mar 10. Acta Biomater. 2018. PMID: 29530821 Free PMC article. Review.
-
A Review of Additive Manufacturing of Biodegradable Fe and Zn Alloys for Medical Implants Using Laser Powder Bed Fusion (LPBF).Materials (Basel). 2024 Dec 19;17(24):6220. doi: 10.3390/ma17246220. Materials (Basel). 2024. PMID: 39769819 Free PMC article. Review.
Cited by
-
Current status and outlook of biodegradable metals in neuroscience and their potential applications as cerebral vascular stent materials.Bioact Mater. 2021 Oct 11;11:140-153. doi: 10.1016/j.bioactmat.2021.09.025. eCollection 2022 May. Bioact Mater. 2021. PMID: 34938919 Free PMC article. Review.
-
Insights into the biocompatibility of biodegradable metallic molybdenum for cardiovascular applications-a critical review.Front Bioeng Biotechnol. 2024 Sep 23;12:1457553. doi: 10.3389/fbioe.2024.1457553. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39376544 Free PMC article. Review.
-
Structure, Biodegradation, and In Vitro Bioactivity of Zn-1%Mg Alloy Strengthened by High-Pressure Torsion.Materials (Basel). 2022 Dec 19;15(24):9073. doi: 10.3390/ma15249073. Materials (Basel). 2022. PMID: 36556879 Free PMC article.
-
Development, Processing and Aging of Novel Zn-Ag-Cu Based Biodegradable Alloys.Materials (Basel). 2023 Apr 18;16(8):3198. doi: 10.3390/ma16083198. Materials (Basel). 2023. PMID: 37110036 Free PMC article.
-
Sutureless vascular anastomotic approaches and their potential impacts.Bioact Mater. 2024 Apr 23;38:73-94. doi: 10.1016/j.bioactmat.2024.04.003. eCollection 2024 Aug. Bioact Mater. 2024. PMID: 38699240 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

