Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications
- PMID: 34027235
- PMCID: PMC8131399
- DOI: 10.1016/j.bioactmat.2021.04.033
Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications
Abstract
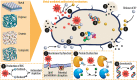
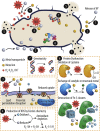
Bacterial infection of implanted scaffolds may have fatal consequences and, in combination with the emergence of multidrug bacterial resistance, the development of advanced antibacterial biomaterials and constructs is of great interest. Since decades ago, metals and their ions had been used to minimize bacterial infection risk and, more recently, metal-based nanomaterials, with improved antimicrobial properties, have been advocated as a novel and tunable alternative. A comprehensive review is provided on how metal ions and ion nanoparticles have the potential to decrease or eliminate unwanted bacteria. Antibacterial mechanisms such as oxidative stress induction, ion release and disruption of biomolecules are currently well accepted. However, the exact antimicrobial mechanisms of the discussed metal compounds remain poorly understood. The combination of different metal ions and surface decorations of nanoparticles will lead to synergistic effects and improved microbial killing, and allow to mitigate potential side effects to the host. Starting with a general overview of antibacterial mechanisms, we subsequently focus on specific metal ions such as silver, zinc, copper, iron and gold, and outline their distinct modes of action. Finally, we discuss the use of these metal ions and nanoparticles in tissue engineering to prevent implant failure.
Keywords: Antibacterial activity; Biomaterials applications; Mechanism of action; Metal ions; Metal nanoparticles; Tissue engineering.
© 2021 The Authors.
Conflict of interest statement
All authors declare no competing financial interests.
Figures





References
-
- Masters E.A., Trombetta R.P., de Mesy Bentley K.L., Boyce B.F., Gill A.L., Gill S.R., Nishitani K., Ishikawa M., Morita Y., Ito H., Bello-Irizarry S.N., Ninomiya M., Brodell J.D., Lee C.C., Hao S.P., Oh I., Xie C., Awad H.A., Daiss J.L., Owen J.R., Kates S.L., Schwarz E.M., Muthukrishnan G. Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy. Bone Res. 2019;7:1–18. doi: 10.1038/s41413-019-0061-z. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

