The effect of normal, metaplastic, and neoplastic esophageal extracellular matrix upon macrophage activation
- PMID: 34027260
- PMCID: PMC8136242
- DOI: 10.1016/j.regen.2020.100037
The effect of normal, metaplastic, and neoplastic esophageal extracellular matrix upon macrophage activation
Abstract
Introduction: Macrophages are capable of extreme plasticity and their activation state has been strongly associated with solid tumor growth progression and regression. Although the macrophage response to extracellular matrix (ECM) isolated from normal tissue is reasonably well understood, there is a relative dearth of information regarding their response to ECM isolated from chronically inflamed tissues, pre-neoplastic tissues, and neoplastic tissues. Esophageal adenocarcinoma (EAC) is a type of neoplasia driven by chronic inflammation in the distal esophagus, and the length of the esophagus provides the opportunity to investigate macrophage behavior in the presence of ECM isolated from a range of disease states within the same organ.
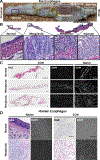
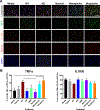
Methods: Normal, metaplastic, and neoplastic ECM hydrogels were prepared from decellularized EAC tissue. The hydrogels were evaluated for their nanofibrous structure (SEM), biochemical profile (targeted and global proteomics), and direct effect upon macrophage (THP-1 cell) activation state (qPCR, ELISA, immunolabeling) and indirect effect upon epithelial cell (Het-1A) migration (Boyden chamber).
Results: Nanofibrous ECM hydrogels from the three tissue types could be formed, and normal and neoplastic ECM showed distinctive protein profiles by targeted and global mass spectroscopy. ECM proteins functionally related to cancer and tumorigenesis were identified in the neoplastic esophageal ECM including collagen alpha-1(VIII) chain (COL8A1), lumican, and elastin. Metaplastic and neoplastic esophageal ECM induce distinctive effects upon THP-1 macrophage signaling compared to normal esophageal ECM. These effects include activation of pro-inflammatory IFNγ and TNFα gene expression and anti-inflammatory IL1RN gene expression. Most notably, neoplastic ECM robustly increased macrophage TNFα protein expression. The secretome of macrophages pre-treated with metaplastic and neoplastic ECM increases the migration of normal esophageal epithelial cells, similar behavior to that shown by tumor cells. Metaplastic ECM shows similar but less pronounced effects than neoplastic ECM suggesting the abnormal signals also exist within the pre-cancerous state.
Conclusion: A progressively diseased ECM, as exists within the esophagus exposed to chronic gastric reflux, can provide insights into novel biomarkers of early disease and identify potential therapeutic targets.
Keywords: Macrophage; decellularization; esophageal adenocarcinoma; extracellular matrix; gastroesophageal reflux; metaplasia.
Conflict of interest statement
Declaration of Interest: LTS, GH, and SFB hold officer positions and have a vested interest in ECM Therapeutics, Inc., which commercializes extracellular matrix materials and components. LH is currently employed by and owns stock in ACell, Inc., which commercializes urinary bladder matrix (UBM) as Gentrix™, MatriStem®, Cytal™, and MicroMatrix®. All other authors declare that they have no competing interests.
Figures



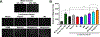
Similar articles
-
Esophageal extracellular matrix hydrogel mitigates metaplastic change in a dog model of Barrett's esophagus.Sci Adv. 2020 Jul 1;6(27):eaba4526. doi: 10.1126/sciadv.aba4526. eCollection 2020 Jul. Sci Adv. 2020. PMID: 32656339 Free PMC article.
-
Expression profiles of cancer stem cell markers: CD133, CD44, Musashi-1 and EpCAM in the cardiac mucosa-Barrett's esophagus-early esophageal adenocarcinoma-advanced esophageal adenocarcinoma sequence.Pathol Res Pract. 2017 Mar;213(3):205-209. doi: 10.1016/j.prp.2016.12.018. Epub 2016 Dec 30. Pathol Res Pract. 2017. PMID: 28216140
-
Esophageal pepsin and proton pump synthesis in barrett's esophagus and esophageal adenocarcinoma.Laryngoscope. 2019 Dec;129(12):2687-2695. doi: 10.1002/lary.28051. Epub 2019 May 2. Laryngoscope. 2019. PMID: 31046139
-
Current state of prognostication, therapy and prospective innovations for Barrett's-related esophageal adenocarcinoma: a literature review.J Gastrointest Oncol. 2021 Aug;12(4):1197-1214. doi: 10.21037/jgo-21-117. J Gastrointest Oncol. 2021. PMID: 34532080 Free PMC article. Review.
-
Impact of the Tumor Microenvironment for Esophageal Tumor Development-An Opportunity for Prevention?Cancers (Basel). 2022 Apr 30;14(9):2246. doi: 10.3390/cancers14092246. Cancers (Basel). 2022. PMID: 35565378 Free PMC article. Review.
Cited by
-
Cardiac matrix-bound Nanovesicles provide insight into mechanisms of clinical heart disease progression to failure.Int J Cardiol. 2025 Feb 15;421:132892. doi: 10.1016/j.ijcard.2024.132892. Epub 2024 Dec 9. Int J Cardiol. 2025. PMID: 39662751 Free PMC article.
-
The Study of the Extracellular Matrix in Chronic Inflammation: A Way to Prevent Cancer Initiation?Cancers (Basel). 2022 Nov 29;14(23):5903. doi: 10.3390/cancers14235903. Cancers (Basel). 2022. PMID: 36497384 Free PMC article. Review.
-
Innovative three-dimensional models for understanding mechanisms underlying lung diseases: powerful tools for translational research.Eur Respir Rev. 2023 Jul 26;32(169):230042. doi: 10.1183/16000617.0042-2023. Print 2023 Sep 30. Eur Respir Rev. 2023. PMID: 37495250 Free PMC article. Review.
-
Matrix-bound nanovesicles alleviate particulate-induced periprosthetic osteolysis.Sci Adv. 2024 Oct 18;10(42):eadn1852. doi: 10.1126/sciadv.adn1852. Epub 2024 Oct 18. Sci Adv. 2024. PMID: 39423278 Free PMC article.
-
Immunomodulatory matrix-bound nanovesicles mitigate acute and chronic pristane-induced rheumatoid arthritis.NPJ Regen Med. 2022 Feb 2;7(1):13. doi: 10.1038/s41536-022-00208-9. NPJ Regen Med. 2022. PMID: 35110573 Free PMC article.
References
-
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A, Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in immunology 23, 549–555 (2002). - PubMed
-
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M, The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology 25, 677–686 (2004). - PubMed
-
- Solinas G, Germano G, Mantovani A, Allavena P, Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. Journal of leukocyte biology 86, 1065–1073 (2009). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
