Exosome-Like Nanoparticles From Lactobacillus rhamnosus GG Protect Against Alcohol-Associated Liver Disease Through Intestinal Aryl Hydrocarbon Receptor in Mice
- PMID: 34027273
- PMCID: PMC8122379
- DOI: 10.1002/hep4.1679
Exosome-Like Nanoparticles From Lactobacillus rhamnosus GG Protect Against Alcohol-Associated Liver Disease Through Intestinal Aryl Hydrocarbon Receptor in Mice
Abstract
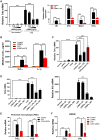
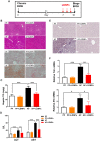
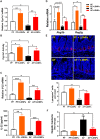
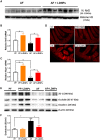
Alcohol-associated liver disease (ALD) is a major cause of mortality. Gut barrier dysfunction-induced bacterial translocation and endotoxin release contribute to the pathogenesis of ALD. Probiotic Lactobacillus rhamnosus GG (LGG) is known to be beneficial on experimental ALD by reinforcing the intestinal barrier function. In this study, we aim to investigate whether the protective effects of LGG on intestinal barrier function is mediated by exosome-like nanoparticles (ELNPs) released by LGG. Intestinal epithelial cells and macrophages were treated with LGG-derived ELNPs (LDNPs) isolated from LGG culture. LDNPs increased tight junction protein expression in epithelial cells and protected from the lipopolysaccharide-induced inflammatory response in macrophages. Three-day oral application of LDNPs protected the intestine from alcohol-induced barrier dysfunction and the liver from steatosis and injury in an animal model of ALD. Co-administration of an aryl hydrocarbon receptor (AhR) inhibitor abolished the protective effects of LDNPs, indicating that the effects are mediated, at least in part, by intestinal AhR signaling. We further demonstrated that LDNP administration increased intestinal interleukin-22-Reg3 and nuclear factor erythroid 2-related factor 2 (Nrf2)-tight junction signaling pathways, leading to the inhibition of bacterial translocation and endotoxin release in ALD mice. This protective effect was associated with LDNP enrichment of bacterial tryptophan metabolites that are AhR agonists. Conclusions: Our results suggest that the beneficial effects of LGG and their supernatant in ALD are likely mediated by bacterial AhR ligand-enriched LDNPs that increase Reg3 and Nrf2 expression, leading to the improved barrier function. These findings provide a strategy for the treatment of ALD and other gut barrier dysfunction-associated diseases.
© 2021 The Authors. Hepatology Communications published by Wiley Periodicals LLC on behalf of the American Association for the Study of Liver Diseases.
Figures



References
-
- Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol 2019;16:605‐616. - PubMed
-
- Knudsen C, Neyrinck AM, Lanthier N, Delzenne NM. Microbiota and nonalcoholic fatty liver disease: promising prospects for clinical interventions? Curr Opin Clin Nutr Metab Care 2019;22:393‐400. - PubMed
-
- Han SH, Suk KT, Kim DJ, Kim MY, Baik SK, Kim YD, et al. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized‐controlled multicenter study. Eur J Gastroenterol Hepatol 2015;27:1300‐1306. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

