Infections at the nexus of metabolic-associated fatty liver disease
- PMID: 34027561
- PMCID: PMC8141380
- DOI: 10.1007/s00204-021-03069-1
Infections at the nexus of metabolic-associated fatty liver disease
Abstract
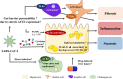
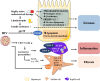
Metabolic-associated fatty liver disease (MAFLD) is a chronic liver disease that affects about a quarter of the world population. MAFLD encompasses different disease stadia ranging from isolated liver steatosis to non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis and hepatocellular carcinoma. Although MAFLD is considered as the hepatic manifestation of the metabolic syndrome, multiple concomitant disease-potentiating factors can accelerate disease progression. Among these risk factors are diet, lifestyle, genetic traits, intake of steatogenic drugs, male gender and particular infections. Although infections often outweigh the development of fatty liver disease, pre-existing MAFLD could be triggered to progress towards more severe disease stadia. These combined disease cases might be underreported because of the high prevalence of both MAFLD and infectious diseases that can promote or exacerbate fatty liver disease development. In this review, we portray the molecular and cellular mechanisms by which the most relevant viral, bacterial and parasitic infections influence the progression of fatty liver disease and steatohepatitis. We focus in particular on how infectious diseases, including coronavirus disease-19, hepatitis C, acquired immunodeficiency syndrome, peptic ulcer and periodontitis, exacerbate MAFLD. We specifically underscore the synergistic effects of these infections with other MAFLD-promoting factors.
Keywords: Helicobacter pylori; Hepatitis C; Human immunodeficiency virus; Infectious diseases; Klebsiella pneumoniae; Lipid metabolism; Liver; Metabolic-associated fatty liver disease (MAFLD); Non-alcoholic steatohepatitis (NASH); SARS-CoV-2.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

