Clinical efficacy of a benazepril and spironolactone combination in dogs with congestive heart failure due to myxomatous mitral valve disease: The BEnazepril Spironolactone STudy (BESST)
- PMID: 34028078
- PMCID: PMC8295662
- DOI: 10.1111/jvim.16155
Clinical efficacy of a benazepril and spironolactone combination in dogs with congestive heart failure due to myxomatous mitral valve disease: The BEnazepril Spironolactone STudy (BESST)
Abstract
Background: The renin-angiotensin-aldosterone system (RAAS), when chronically activated, is harmful and RAAS-suppressive drugs are beneficial in the treatment of congestive heart failure (CHF). Mineralocorticoid receptor antagonists are widely used in the treatment of CHF in people.
Hypothesis/objectives: To determine if a mineralocorticoid receptor antagonist (spironolactone) is beneficial and safe in CHF due to myxomatous mitral valve disease (MMVD) of varying severity, we hypothesized that, when combined with furosemide, a combination product (S+BNZ) containing the ACE inhibitor (ACE-I), benazepril, and spironolactone, would be superior to benazepril alone.
Animals: Five hundred and sixty-nine client-owned dogs, with MMVD and CHF (ACVIM Stage C) of ≤10-days' duration.
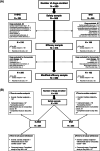
Methods: After initial stabilization, dogs were randomized into a positive-controlled, double-blind, multicenter trial, to receive furosemide plus S+BNZ or furosemide plus benazepril. The primary outcome variable was the percentage of dogs reaching cardiac endpoint before Day 360. Cardiac endpoint was defined as cardiac death or euthanasia, recurrence of pulmonary edema, necessity for nonauthorized cardiac drug(s) or a furosemide dosage >8 mg/kg/d.
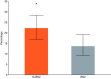
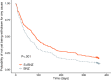
Results: A significantly lower percentage of dogs treated with S+BNZ reached the primary outcome variable by Day 360 (OR = 0.56; 95% CI, 0.32-0.98; P = .04) and risk of dying or worsening from cardiac causes, was significantly reduced (HR = 0.73; 95% CI = 0.59-0.89, P = .002) vs benazepril alone. Adverse events, potentially associated with treatment, were rare and equal between groups.
Conclusion and clinical importance: The combination of S+BNZ is effective, safe, and superior to benazepril alone, when used with furosemide for the management of mild, moderate or severe CHF caused by MMVD in dogs.
Keywords: ACE inhibitor; MRA; RAAS; aldosterone breakthrough; mitral regurgitation.
© 2021 Ceva Animal Health, LLC. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Melissa Coffman, Emilie Guillot, Thomas Blondel, Catherine Garelli‐Paar, Shuo Feng and Susanne Heartsill are employees of Ceva Santé Animale. During the past 5 years, Clarke E. Atkins has received research funding, reimbursement for travel, honoraria for speaking and preparation of educational materials, and consulting fees from Ceva Santé Animale, Boehringer Ingelheim Animal Health GmbH, Vetoquinol, and Virbac, all of whom market cardiac drugs. This manuscript describes the effects of a drug manufactured by Ceva Santé Animale.
Figures




References
-
- Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486‐492. - PubMed
-
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709‐717. - PubMed
-
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Eur Heart J. 2012;33(14):1787‐1847. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guidelines for the management of heart failure. Circulation. 2013;128:e240‐e327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

