Efficacy of Neratinib Plus Capecitabine in the Subgroup of Patients with Central Nervous System Involvement from the NALA Trial
- PMID: 34028126
- PMCID: PMC8342591
- DOI: 10.1002/onco.13830
Efficacy of Neratinib Plus Capecitabine in the Subgroup of Patients with Central Nervous System Involvement from the NALA Trial
Abstract
Background: Neratinib has efficacy in central nervous system (CNS) metastases from HER2-positive metastatic breast cancer (MBC). We report outcomes among patients with CNS metastases at baseline from the phase III NALA trial of neratinib plus capecitabine (N + C) versus lapatinib plus capecitabine (L + C).
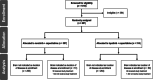
Materials and methods: NALA was a randomized, active-controlled trial in patients who received two or more previous HER2-directed regimens for HER2-positive MBC. Patients with asymptomatic/stable brain metastases (treated or untreated) were eligible. Patients were assigned to N + C (neratinib 240 mg per day, capecitabine 750 mg/m2 twice daily) or L + C (lapatinib 1,250 mg per day, capecitabine 1,000 mg/m2 twice daily) orally. Independently adjudicated progression-free survival (PFS), overall survival (OS), and CNS endpoints were considered.
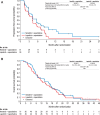
Results: Of 621 patients enrolled, 101 (16.3%) had known CNS metastases at baseline (N + C, n = 51; L + C, n = 50); 81 had received prior CNS-directed radiotherapy and/or surgery. In the CNS subgroup, mean PFS through 24 months was 7.8 months with N + C versus 5.5 months with L + C (hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.41-1.05), and mean OS through 48 months was 16.4 versus 15.4 months (HR, 0.90; 95% CI, 0.59-1.38). At 12 months, cumulative incidence of interventions for CNS disease was 25.5% for N + C versus 36.0% for L + C, and cumulative incidence of progressive CNS disease was 26.2% versus 41.6%, respectively. In patients with target CNS lesions at baseline (n = 32), confirmed intracranial objective response rates were 26.3% and 15.4%, respectively. No new safety signals were observed.
Conclusion: These analyses suggest improved PFS and CNS outcomes with N + C versus L + C in patients with CNS metastases from HER2-positive MBC.
Implications for practice: In a subgroup of patients with central nervous system (CNS) metastases from HER2-positive breast cancer after two or more previous HER2-directed regimens, the combination of neratinib plus capecitabine was associated with improved progression-free survival and CNS outcomes compared with lapatinib plus capecitabine. These findings build on previous phase II and III studies describing efficacy of neratinib in the prevention and treatment of CNS metastases, and support a role for neratinib as a systemic treatment option in the management of patients with HER2-positive brain metastases following antibody-based HER2-directed therapies.
Keywords: Capecitabine; Central nervous system neoplasms; Lapatinib; Neratinib; Receptor, ErbB-2.
© 2021 The Authors. The Oncologist published by Wiley Periodicals LLC on behalf of AlphaMed Press.
Conflict of interest statement
Figures


References
-
- Lim E, Lin NU. Updates on the management of breast cancer brain metastases. Oncology (Williston Park) 2014;28:572–578. - PubMed
-
- von Minckwitz G, Huang CS, Mano MS et al. Trastuzumab emtansine for residual invasive HER2‐positive breast cancer. N Engl J Med 2019;380:617–628. - PubMed
-
- Piccart M, Procter M, Fumagalli D et al. GS1‐04. Interim overall survival analysis of APHINITY (BIG 4‐11): A randomized multicenter, double‐blind, placebo‐controlled trial comparing chemotherapy plus trastuzumab plus pertuzumab versus chemotherapy plus trastuzumab plus placebo as adjuvant therapy in patients with operable HER2‐positive early breast cancer. Cancer Res 2020;80(4 suppl):GS1‐04a.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

