Introduction to the EQIPD quality system
- PMID: 34028353
- PMCID: PMC8184207
- DOI: 10.7554/eLife.63294
Introduction to the EQIPD quality system
Abstract
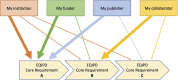
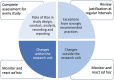
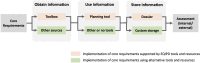
While high risk of failure is an inherent part of developing innovative therapies, it can be reduced by adherence to evidence-based rigorous research practices. Supported through the European Union's Innovative Medicines Initiative, the EQIPD consortium has developed a novel preclinical research quality system that can be applied in both public and private sectors and is free for anyone to use. The EQIPD Quality System was designed to be suited to boost innovation by ensuring the generation of robust and reliable preclinical data while being lean, effective and not becoming a burden that could negatively impact the freedom to explore scientific questions. EQIPD defines research quality as the extent to which research data are fit for their intended use. Fitness, in this context, is defined by the stakeholders, who are the scientists directly involved in the research, but also their funders, sponsors, publishers, research tool manufacturers, and collaboration partners such as peers in a multi-site research project. The essence of the EQIPD Quality System is the set of 18 core requirements that can be addressed flexibly, according to user-specific needs and following a user-defined trajectory. The EQIPD Quality System proposes guidance on expectations for quality-related measures, defines criteria for adequate processes (i.e. performance standards) and provides examples of how such measures can be developed and implemented. However, it does not prescribe any pre-determined solutions. EQIPD has also developed tools (for optional use) to support users in implementing the system and assessment services for those research units that successfully implement the quality system and seek formal accreditation. Building upon the feedback from users and continuous improvement, a sustainable EQIPD Quality System will ultimately serve the entire community of scientists conducting non-regulated preclinical research, by helping them generate reliable data that are fit for their intended use.
Keywords: drug discovery; neuroscience; nonregulated research; research rigor.
© 2021, Bespalov et al.
Conflict of interest statement
AB AB is an employee and/or shareholder at PAASP GmbH, PAASP US LLC, Exciva GmbH, Synventa LLC, Ritec Pharma, RB, MA, LB, Nd, EC, AD, RF, VG, SH, MH, MK, MP, PP, EP, LR, GR, MR, JS, MS, CS, VV, JV, KW No competing interests declared, AG AG are employees of Janssen / Johnson & Johnson and shareholders at Johnson & Johnson, BG, CE BG and CE are employees and shareholders at PAASP GmbH. JG JG is an employee of AAALAC International that is an EQIPD Associated Collaborator. VC, CF VC and CFC are employees of Porsolt. IL, FD IAL and FD are employees of Sanofi. LM LM is an employee and shareholder of UCB. SB SB is an employee of Novartis Pharma. BA BA is an employee and shareholder of Pfizer. The views and opinions expressed in this article are those of the individual author and should not be attributed to Pfizer, its directors, officers, employees, affiliates, or any organization with which the author is employed or affiliated. CF AB, BA, NdB, UD, CFB, PK, MK, MM, PM, PP, GR, JS, and TS are members of the Preclinical Data Forum (co-chairs - AB and TS), a network financially and organizationally supported by ECNP and Cohen Veterans Bioscience. PK PK is an employee and shareholder at PAASP US LLC. CK UD and CK receive funding from Volkswagen Foundation, PM PM is owner of Cerbascience Consulting, HP HP has received during the last three years consulting and speaking fees and/or funding for collaborative projects from Bayer, Roche, Zogenix, and Eisai. KW KW is a consultant of Avertim, Brussels, Belgium, support for this contribution was funded by Janssen Pharmaceutica NV. MM MM, UD and TS are members of the Advisory Board at PAASP. MM, UD and TS are members of the ARRIVE guidelines working group. UD UD and CK receive funding from Volkswagen Foundation. MM, UD and TS are members of the Advisory Board at PAASP. MM, UD and TS are members of the ARRIVE guidelines working group. TS MM, UD and TS are members of the Advisory Board at PAASP. MM, UD and TS are members of the ARRIVE guidelines working group. TS is an AAALAC ad-hoc specialist. TS and AG are employees of Janssen / Johnson & Johnson and shareholders at Johnson & Johnson.
Figures


References
-
- Bespalov A, Steckler T, Altevogt B, Koustova E, Skolnick P, Deaver D, Millan MJ, Bastlund JF, Doller D, Witkin J, Moser P, O'Donnell P, Ebert U, Geyer MA, Prinssen E, Ballard T, Macleod M. Failed trials for central nervous system disorders do not necessarily invalidate preclinical models and drug targets. Nature Reviews Drug Discovery. 2016;15:516. doi: 10.1038/nrd.2016.88. - DOI - PubMed
-
- Deming WE. Ocut of the Crisis. Massachusetts Institute of Technology, Center for Advanced Engineering Study; 1986.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

