Immunogenicity and Reactogenicity After SARS-CoV-2 mRNA Vaccination in Kidney Transplant Recipients Taking Belatacept
- PMID: 34028386
- PMCID: PMC8380692
- DOI: 10.1097/TP.0000000000003824
Immunogenicity and Reactogenicity After SARS-CoV-2 mRNA Vaccination in Kidney Transplant Recipients Taking Belatacept
Abstract
Background: Belatacept may impair humoral immunity, impacting the effectiveness of SARS-CoV-2 mRNA vaccines in transplant recipients. We investigated immunogenicity after SARS-CoV-2 mRNA vaccines in kidney transplant recipients who are and are not taking belatacept.
Methods: Participants were recruited between December 9, 2020, and April 1, 2021. Blood samples were collected after dose 1 and dose 2 (D1, D2) and analyzed using either an anti-SARS-CoV-2 enzyme immunoassay against the S1 domain of the SARS-CoV-2 spike protein or immunoassay against the receptor-binding domain of the SARS-CoV-2 spike protein. Stabilized inverse probability of treatment weights was used to compare immunogenicity, and a weighted logistics regression was used to calculate fold change of positive response.
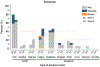
Results: Among the 609 participants studied, 24 (4%) were taking belatacept. After dose 1, 0/24 (0%) belatacept patients had detectable antibodies, compared with 77 of 568 (14%) among the equivalent nonbelatacept population (P = 0.06). After dose 2, 1/19 (5%) belatacept patients had detectable antibodies, compared with 190/381 (50%) among the equivalent nonbelatacept population (P < 0.001). Belatacept use was associated with 16.7-fold lower odds of having a positive post-D2 titer result (P < 0.01).
Conclusions: Additional measures need to be explored to protect kidney transplant recipients taking belatacept. Best safety practices should be continued despite vaccination among this population.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
DISCLOSURES
The authors of this manuscript have the following conflicts of interest to disclose as described by
Figures
References
-
- Chavarot N et al.Poor Anti-SARS-CoV-2 Humoral and T-cell Responses After 2 Injections of mRNA Vaccine in Kidney Transplant Recipients Treated with Belatacept. Transplantation Online First, (2021). - PubMed
-
- Leibler C et al.Kidney transplant recipients treated with belatacept exhibit increased naïve and transitional B cells. Am J Transplant 14, 1173–1182 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous