Safety of Early Discontinuation of Antiseizure Medication After Acute Symptomatic Neonatal Seizures
- PMID: 34028496
- PMCID: PMC8145161
- DOI: 10.1001/jamaneurol.2021.1437
Safety of Early Discontinuation of Antiseizure Medication After Acute Symptomatic Neonatal Seizures
Erratum in
-
CC-BY Open Access Added.JAMA Neurol. 2021 Jul 1;78(7):882. doi: 10.1001/jamaneurol.2021.2227. JAMA Neurol. 2021. PMID: 34236400 Free PMC article. No abstract available.
Abstract
Importance: Antiseizure medication (ASM) treatment duration for acute symptomatic neonatal seizures is variable. A randomized clinical trial of phenobarbital compared with placebo after resolution of acute symptomatic seizures closed early owing to low enrollment.
Objective: To assess whether ASM discontinuation after resolution of acute symptomatic neonatal seizures and before hospital discharge is associated with functional neurodevelopment or risk of epilepsy at age 24 months.
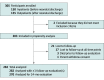
Design, setting, and participants: This comparative effectiveness study included 303 neonates with acute symptomatic seizures (282 with follow-up data and 270 with the primary outcome measure) from 9 US Neonatal Seizure Registry centers, born from July 2015 to March 2018. The centers all had level IV neonatal intensive care units and comprehensive pediatric epilepsy programs. Data were analyzed from June 2020 to February 2021.
Exposures: The primary exposure was duration of ASM treatment dichotomized as ASM discontinued vs ASM maintained at the time of discharge from the neonatal seizure admission. To enhance causal association, each outcome risk was adjusted for propensity to receive ASM at discharge. Propensity for ASM maintenance was defined by a logistic regression model including seizure cause, gestational age, therapeutic hypothermia, worst electroencephalogram background, days of electroencephalogram seizures, and discharge examination (all P ≤ .10 in a joint model except cause, which was included for face validity).
Main outcomes and measures: Functional neurodevelopment was assessed by the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA-FS) at 24 months powered for propensity-adjusted noninferiority of early ASM discontinuation. Postneonatal epilepsy, a prespecified secondary outcome, was defined per International League Against Epilepsy criteria, determined by parent interview, and corroborated by medical records.
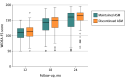
Results: Most neonates (194 of 303 [64%]) had ASM maintained at the time of hospital discharge. Among 270 children evaluated at 24 months (mean [SD], 23.8 [0.7] months; 147 [54%] were male), the WIDEA-FS score was similar for the infants whose ASMs were discontinued (101 of 270 [37%]) compared with the infants with ASMs maintained (169 of 270 [63%]) at discharge (median score, 165 [interquartile range, 150-175] vs 161 [interquartile range, 129-174]; P = .09). The propensity-adjusted average difference was 4 points (90% CI, -3 to 11 points), which met the a priori noninferiority limit of -12 points. The epilepsy risk was similar (11% vs 14%; P = .49), with a propensity-adjusted odds ratio of 1.5 (95% CI, 0.7-3.4; P = .32).
Conclusions and relevance: In this comparative effectiveness study, no difference was found in functional neurodevelopment or epilepsy at age 24 months among children whose ASM was discontinued vs maintained at hospital discharge after resolution of acute symptomatic neonatal seizures. These results support discontinuation of ASM prior to hospital discharge for most infants with acute symptomatic neonatal seizures.
Conflict of interest statement
Figures

Comment in
-
Discontinuing Antiseizure Medication in Neonates With Acute Symptomatic Seizures-Primum non nocere.JAMA Neurol. 2021 Jul 1;78(7):797-799. doi: 10.1001/jamaneurol.2021.1218. JAMA Neurol. 2021. PMID: 34028499 No abstract available.
-
Safety of Early Discontinuation of Antiseizure Medication After Acute Symptomatic Neonatal Seizures-Reply.JAMA Neurol. 2022 Jan 1;79(1):91-92. doi: 10.1001/jamaneurol.2021.4112. JAMA Neurol. 2022. PMID: 34779827 No abstract available.
-
Safety of Early Discontinuation of Antiseizure Medication After Acute Symptomatic Neonatal Seizures.JAMA Neurol. 2022 Jan 1;79(1):90-91. doi: 10.1001/jamaneurol.2021.4109. JAMA Neurol. 2022. PMID: 34779830 No abstract available.
-
EBNEO Commentary: Safety of early discontinuation of anti-seizure medication in neonates.Acta Paediatr. 2022 Feb;111(2):449-450. doi: 10.1111/apa.16200. Epub 2021 Dec 15. Acta Paediatr. 2022. PMID: 34907580 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

