Common molecular mechanisms of SLC6A1 variant-mediated neurodevelopmental disorders in astrocytes and neurons
- PMID: 34028503
- PMCID: PMC8418336
- DOI: 10.1093/brain/awab207
Common molecular mechanisms of SLC6A1 variant-mediated neurodevelopmental disorders in astrocytes and neurons
Abstract
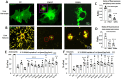
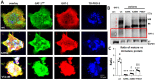
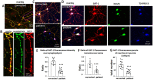
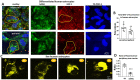
Solute carrier family 6 member 1 (SLC6A1) is abundantly expressed in the developing brain even before the CNS is formed. Its encoded GABA transporter 1 (GAT-1) is responsible for the reuptake of GABA into presynaptic neurons and glia, thereby modulating neurotransmission. GAT-1 is expressed globally in the brain, in both astrocytes and neurons. The GABA uptake function of GAT-1 in neurons cannot be compensated for by other GABA transporters, while the function in glia can be partially replaced by GABA transporter 3. Recently, many variants in SLC6A1 have been associated with a spectrum of epilepsy syndromes and neurodevelopmental disorders, including myoclonic atonic epilepsy, childhood absence epilepsy, autism, and intellectual disability, but the pathomechanisms associated with these phenotypes remain unclear. The presence of GAT-1 in both neurons and astrocytes further obscures the role of abnormal GAT-1 in the heterogeneous disease phenotype manifestations. Here we examine the impact on transporter trafficking and function of 22 SLC6A1 variants identified in patients with a broad spectrum of phenotypes. We also evaluate changes in protein expression and subcellular localization of the variant GAT-1 in various cell types, including neurons and astrocytes derived from human patient induced pluripotent stem cells. We found that a partial or complete loss-of-function represents a common disease mechanism, although the extent of GABA uptake reduction is variable. The reduced GABA uptake appears to be due to reduced cell surface expression of the variant transporter caused by variant protein misfolding, endoplasmic reticulum retention, and subsequent degradation. Although the extent of reduction of the total protein, surface protein, and the GABA uptake level of the variant transporters is variable, the loss of GABA uptake function and endoplasmic reticulum retention is consistent across induced pluripotent stem cell-derived cell types, including astrocytes and neurons, for the surveyed variants. Interestingly, we did not find a clear correlation of GABA uptake function and the disease phenotypes, such as myoclonic atonic epilepsy versus developmental delay, in this study. Together, our study suggests that impaired transporter protein trafficking and surface expression are the major disease-associated mechanisms associated with pathogenic SLC6A1 variants. Our results resemble findings from pathogenic variants in other genes affecting the GABA pathway, such as GABAA receptors. This study provides critical insight into therapeutic developments for SLC6A1 variant-mediated disorders and implicates that boosting transporter function by either genetic or pharmacological approaches would be beneficial.
Keywords: ER retention; GABA transporter 1 (GAT-1); SLC6A1; autism; epilepsy.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures




Comment in
-
Pathogenic mechanisms underlying SLC6A1 variant-mediated neurodevelopmental disorders.Brain. 2021 Sep 4;144(8):2237-2239. doi: 10.1093/brain/awab259. Brain. 2021. PMID: 34283886 No abstract available.
References
-
- Ben-Ari Y.Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–739. - PubMed
-
- Andang M, Hjerling-Leffler J, Moliner A, et al. Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature. 2008;451(7177):460–464. - PubMed
-
- Ge S, Pradhan DA, Ming GL, Song H.. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30(1):1–8. - PubMed
-
- Loo DD, Eskandari S, Boorer KJ, Sarkar HK, Wright EM.. Role of Cl- in electrogenic Na+-coupled cotransporters GAT1 and SGLT1. J Biol Chem. 2000;275(48):37414–37422. - PubMed
-
- Sacher A, Nelson N, Ogi JT, Wright EM, Loo DD, Eskandari S.. Presteady-state and steady-state kinetics and turnover rate of the mouse gamma-aminobutyric acid transporter (mGAT3). J Membr Biol. 2002;190(1):57–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

