Plasma levels of angiopoietin-2, VEGF-A, and VCAM-1 as markers of bevacizumab-induced hypertension: CALGB 80303 and 90401 (Alliance)
- PMID: 34028627
- PMCID: PMC8611102
- DOI: 10.1007/s10456-021-09799-1
Plasma levels of angiopoietin-2, VEGF-A, and VCAM-1 as markers of bevacizumab-induced hypertension: CALGB 80303 and 90401 (Alliance)
Abstract
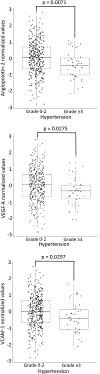
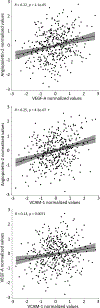
Hypertension is a common toxicity induced by bevacizumab and other antiangiogenic drugs. There are no biomarkers to predict the risk of bevacizumab-induced hypertension. This study aimed to identify plasma proteins related to the function of the vasculature to predict the risk of severe bevacizumab-induced hypertension. Using pretreated plasma samples from 398 bevacizumab-treated patients in two clinical trials (CALGB 80303 and 90401), the levels of 17 proteins were measured via ELISA. The association between proteins and grade 3 bevacizumab-induced hypertension was performed by calculating the odds ratio (OR) from logistic regression adjusting for age, sex, and clinical trial. Using the optimal cut-point of each protein, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for hypertension were estimated. Five proteins showed no difference in levels between clinical trials and were used for analyses. Lower levels of angiopoietin-2 (p = 0.0013, OR 3.41, 95% CI 1.67-7.55), VEGF-A (p = 0.0008, OR 4.25, 95% CI 1.93-10.72), and VCAM-1 (p = 0.0067, OR 2.68, 95% CI 1.34-5.63) were associated with an increased risk of grade 3 hypertension. The multivariable model suggests independent effects of angiopoietin-2 (p = 0.0111, OR 2.71, 95% CI 1.29-6.10), VEGF-A (p = 0.0051, OR 3.66, 95% CI 1.54-9.73), and VCAM-1 (p = 0.0308, OR 2.27, 95% CI 1.10-4.92). The presence of low levels of 2-3 proteins had an OR of 10.06 (95% CI 3.92-34.18, p = 1.80 × 10-5) for the risk of hypertension, with sensitivity of 89.7%, specificity of 53.5%, PPV of 17.3%, and NPV of 97.9%. This is the first study providing evidence of plasma proteins with potential value to predict patients at risk of developing bevacizumab-induced hypertension.Clinical trial registration: ClinicalTrials.gov Identifier: NCT00088894 (CALGB 80303); and NCT00110214 (CALGB 90401).
Keywords: Angiopoietin-2; Bevacizumab; Hypertension; VCAM-1; VEGF-A.
© 2021. The Author(s), under exclusive licence to Springer Nature B.V.
Conflict of interest statement
Declarations
Figures


References
-
- Garcia J, Hurwitz HI, Sandler AB et al. (2020). Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev 86:102017. - PubMed
-
- EMA Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/avastin-epar-.... Accessed 05 October 2020.
-
- FDA AVASTIN®Prescribing Information: Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125085s332lbl.pdf. Accessed 05 October 2020.
-
- Quintanilha JCF, Wang J, Sibley AB et al. (2020). Bevacizumab-induced hypertension and proteinuria: A genome-wide analysis of more than 1,000 patients. Submitted to Br J Cancer.
-
- Moslehi JJ (2016). Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 375:1457–1467. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1CA233327/Foundation for the National Institutes of Health (US)
- U10CA180821/National Cancer Institute of the National Institutes of Health
- UG1CA233253/National Cancer Institute of the National Institutes of Health
- UG1 CA233373/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- UG1CA233373/National Cancer Institute of the National Institutes of Health
- UG1CA233337/National Cancer Institute of the National Institutes of Health
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA233337/CA/NCI NIH HHS/United States
- U24 CA196171/CA/NCI NIH HHS/United States
- UG1 CA233327/CA/NCI NIH HHS/United States
- U24CA196171/National Cancer Institute of the National Institutes of Health
- UG1 CA233253/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

