Enhanced Delivery of Neuroactive Drugs via Nasal Delivery with a Self-Healing Supramolecular Gel
- PMID: 34029010
- PMCID: PMC8292877
- DOI: 10.1002/advs.202101058
Enhanced Delivery of Neuroactive Drugs via Nasal Delivery with a Self-Healing Supramolecular Gel
Abstract
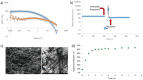
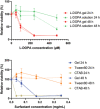
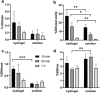
This paper reports the use of a self-assembling hydrogel as a delivery vehicle for the Parkinson's disease drug l-DOPA. Based on a two-component combination of an l-glutamine amide derivative and benzaldehyde, this gel has very soft rheological properties and self-healing characteristics. It is demonstrated that the gel can be formulated to encapsulate l-DOPA. These drug-loaded gels are characterized, and rapid release of the drug is obtained from the gel network. This drug-loaded hydrogel has appropriate rheological characteristics to be amenable for injection. This system is therefore tested as a vehicle for nasal delivery of neurologically-active drugs-a drug delivery strategy that can potentially avoid first pass liver metabolism and bypass the blood-brain barrier, hence enhancing brain uptake. In vitro tests indicate that the gel has biocompatibility with respect to nasal epithelial cells. Furthermore, animal studies demonstrate that the nasal delivery of a gel loaded with 3 H-labeled l-DOPA out-performed a simple intranasal l-DOPA solution. This is attributed to longer residence times of the gel in the nasal cavity resulting in increased blood and brain concentrations. It is demonstrated that the likely routes of brain penetration of intranasally-delivered l-DOPA gel involve the trigeminal and olfactory nerves connecting to other brain regions.
Keywords: drug delivery; hydrogel; neuroactive drugs, supramolecular gel.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- a) Sangeetha N. M., Maitra U., Chem. Soc. Rev. 2005, 34, 821; - PubMed
- b) Hirst A. R., Escuder B., Miravet J. F., Smith D. K., Angew. Chem., Int. Ed. 2008, 47, 8002; - PubMed
- c) Du X., Zhou J., Shi J., Xu B., Chem. Rev. 2015, 115, 13165; - PMC - PubMed
- d) Smith D. K., in Molecular Gels: Structure and Dynamics (Ed. Weiss R. G.), Royal Society of Chemistry, Cambridge: 2018, pp. 300–371.
-
- a) Verma G., Hassan P. A., Phys. Chem. Chem. Phys. 2013, 15, 17016; - PubMed
- b) Skilling K. J., Citossi F., Bradshaw T. D., Ashford M., Kellam B., Marlow M., Soft Matter 2014, 10, 237; - PubMed
- c) Vashist A., Kaushik A., Alexis K., Jayant R. D., Sagar V., Vashist A., Nair M., Curr. Pharm. Des. 2017, 23, 3595; - PMC - PubMed
- d) Mayr J., Saldías C., Díaz D. D., Chem. Soc. Rev. 2018, 47, 1484. - PubMed
- e) Esposito C. L., Kirilov P., Roullin V. G., J. Controlled Release 2018, 271, 1. - PubMed
- f) Dastidar P., Roy R., Parveen R., Sarkar K., Adv. Ther. 2019, 2, 1800061.
-
- a) Valéry C., Paternostre M., Robert B., Gulik‐Krzywicki T., Narayanan T., Dedieu J.‐C., Keller G., Torres M.‐L., Cherif‐Cheikh R., Calvo P., Artzner F., Proc. Natl. Acad. Sci., U. S. A. 2003, 100, 10258; - PMC - PubMed
- b) Yang Z., Gu H., Zhang Y., Xu B., Chem. Commun. 2004, 208; - PubMed
- c) Friggeri A., Feringa B. L., van Esch J., J. Controlled Release 2004, 97, 241; - PubMed
- d) Vemula P. K., Cruikshank G. A., Karp J. M., John G., Biomaterials 2009, 30, 383; - PubMed
- e) Gao Y., Kuang Y., Guo Z., Guo Z., Krauss I. J., Xu B., J. Am. Chem. Soc. 2009, 131, 13576; - PubMed
- f) Matsona J. B., Stupp S. I., Chem. Commun. 2011, 47, 7962; - PMC - PubMed
- g) Milanesi L., Hunter C. A., Tzokova N., Waltho J. P., Tomas S., Chem. ‐ Eur. J. 2011, 17, 9753; - PubMed
- h) Saha S., Bachl J., Kundu T., Díaz D. D., Banerjee R., Chem. Commun. 2014, 50, 7032; - PubMed
- i) Roy R., Deb J., Jana S., Dastidar P., Chem. ‐ Eur. J. 2014, 20, 15320; - PubMed
- j) Truong P. T. W. T., Su Y., Gloria D., Braet F., Truong W. T., Su Y., Gloria D., Braet F., Thordarson P., Biomater. Sci. 2015, 3, 298; - PubMed
- k) Howe E. J., Okesola B. O., Smith D. K., Chem. Commun. 2015, 51, 7451; - PubMed
- l) Patterson A. K., Smith D. K., Chem. Commun. 2020, 56, 11046. - PubMed
-
- a) Cao S., Fu X., Wang N., Wang H., Yang Y., Int. J. Pharm. 2008, 357, 95; - PubMed
- b) Panda J. J., Mishra A., Basu A., Chauhan V. S., Biomacromolecules 2008, 9, 2244; - PubMed
- c) Thota C. K., Yadav N., Chauhan V. S., Sci. Rep. 2016, 6, 31167; - PMC - PubMed
- d) Cinar G., Ozdemir A., Hamsici S., Gunay G., Dana A., Tekinay A. B., Guler M. O., Biomater. Sci. 2017, 5, 67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
