Vitamin D supplementation for the treatment of COVID-19: a living systematic review
- PMID: 34029377
- PMCID: PMC8406457
- DOI: 10.1002/14651858.CD015043
Vitamin D supplementation for the treatment of COVID-19: a living systematic review
Abstract
Background: The role of vitamin D supplementation as a treatment for COVID-19 has been a subject of considerable discussion. A thorough understanding of the current evidence regarding the effectiveness and safety of vitamin D supplementation for COVID-19 based on randomised controlled trials is required.
Objectives: To assess whether vitamin D supplementation is effective and safe for the treatment of COVID-19 in comparison to an active comparator, placebo, or standard of care alone, and to maintain the currency of the evidence, using a living systematic review approach.
Search methods: We searched the Cochrane COVID-19 Study Register, Web of Science and the WHO COVID-19 Global literature on coronavirus disease to identify completed and ongoing studies without language restrictions to 11 March 2021.
Selection criteria: We followed standard Cochrane methodology. We included randomised controlled trials (RCTs) evaluating vitamin D supplementation for people with COVID-19, irrespective of disease severity, age, gender or ethnicity. We excluded studies investigating preventive effects, or studies including populations with other coronavirus diseases (severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS)).
Data collection and analysis: We followed standard Cochrane methodology. To assess bias in included studies, we used the Cochrane risk of bias tool (ROB 2) for RCTs. We rated the certainty of evidence using the GRADE approach for the following prioritised outcome categories: individuals with moderate or severe COVID-19: all-cause mortality, clinical status, quality of life, adverse events, serious adverse events, and for individuals with asymptomatic or mild disease: all-cause mortality, development of severe clinical COVID-19 symptoms, quality of life, adverse events, serious adverse events.
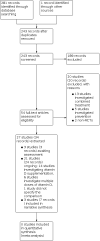
Main results: We identified three RCTs with 356 participants, of whom 183 received vitamin D. In accordance with the World Health Organization (WHO) clinical progression scale, two studies investigated participants with moderate or severe disease, and one study individuals with mild or asymptomatic disease. The control groups consisted of placebo treatment or standard of care alone. Effectiveness of vitamin D supplementation for people with COVID-19 and moderate to severe disease We included two studies with 313 participants. Due to substantial clinical and methodological diversity of both studies, we were not able to pool data. Vitamin D status was unknown in one study, whereas the other study reported data for vitamin D deficient participants. One study administered multiple doses of oral calcifediol at days 1, 3 and 7, whereas the other study gave a single high dose of oral cholecalciferol at baseline. We assessed one study with low risk of bias for effectiveness outcomes, and the other with some concerns about randomisation and selective reporting. All-cause mortality at hospital discharge (313 participants) We found two studies reporting data for this outcome. One study reported no deaths when treated with vitamin D out of 50 participants, compared to two deaths out of 26 participants in the control group (Risk ratio (RR) 0.11, 95% confidence interval (CI) 0.01 to 2.13). The other study reported nine deaths out of 119 individuals in the vitamin D group, whereas six participants out of 118 died in the placebo group (RR 1.49, 95% CI 0.55 to 4.04]. We are very uncertain whether vitamin D has an effect on all-cause mortality at hospital discharge (very low-certainty evidence). Clinical status assessed by the need for invasive mechanical ventilation (237 participants) We found one study reporting data for this outcome. Nine out of 119 participants needed invasive mechanical ventilation when treated with vitamin D, compared to 17 out of 118 participants in the placebo group (RR 0.52, 95% CI 0.24 to 1.13). Vitamin D supplementation may decrease need for invasive mechanical ventilation, but the evidence is uncertain (low-certainty evidence). Quality of life We did not find data for quality of life. Safety of vitamin D supplementation for people with COVID-19 and moderate to severe disease We did not include data from one study, because assessment of serious adverse events was not described and we are concerned that data might have been inconsistently measured. This study reported vomiting in one out of 119 participants immediately after vitamin D intake (RR 2.98, 95% CI 0.12 to 72.30). We are very uncertain whether vitamin D supplementation is associated with higher risk for adverse events (very low-certainty). Effectiveness and safety of vitamin D supplementation for people with COVID-19 and asymptomatic or mild disease We found one study including 40 individuals, which did not report our prioritised outcomes, but instead data for viral clearance, inflammatory markers, and vitamin D serum levels. The authors reported no events of hypercalcaemia, but recording and assessment of further adverse events remains unclear. Authors administered oral cholecalciferol in daily doses for at least 14 days, and continued with weekly doses if vitamin D blood levels were > 50 ng/mL.
Authors' conclusions: There is currently insufficient evidence to determine the benefits and harms of vitamin D supplementation as a treatment of COVID-19. The evidence for the effectiveness of vitamin D supplementation for the treatment of COVID-19 is very uncertain. Moreover, we found only limited safety information, and were concerned about consistency in measurement and recording of these outcomes. There was substantial clinical and methodological heterogeneity of included studies, mainly because of different supplementation strategies, formulations, vitamin D status of participants, and reported outcomes. There is an urgent need for well-designed and adequately powered randomised controlled trials (RCTs) with an appropriate randomisation procedure, comparability of study arms and preferably double-blinding. We identified 21 ongoing and three completed studies without published results, which indicates that these needs will be addressed and that our findings are subject to change in the future. Due to the living approach of this work, we will update the review periodically.
Trial registration: ClinicalTrials.gov NCT04366908 NCT04449718 NCT04459247.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
JS: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project "CEOSys", which was paid to the institution).
JW: none known
CI: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project "CEOSys", which was paid to the institution).
AM: none known
CB: none known
MIM: is member of the CEOsys project funded by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021, paid to the institution.
PM: institution received grants by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF), grant number 01KG1815, for the VITDALIZE‐Study.
MB: none known
NS: none known
MS: none known
VP: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project "CEOSys", which was paid to the institution).
Figures










Comment in
-
Vitamin D supplementation for the treatment of COVID-19: Summary of a living Cochrane review.Explore (NY). 2021 Sep-Oct;17(5):481-482. doi: 10.1016/j.explore.2021.06.004. Epub 2021 Jun 27. Explore (NY). 2021. PMID: 34294560 Free PMC article. No abstract available.
References
References to studies included in this review
Entrenas Castillo 2020 {published data only}
-
- Entrenas Castillo E, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Diaz JF, López Miranda J, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus bestavailable therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. Journal of Steroid Biochemistry and Molecular Biology 2020;203:105-751. [DOI: 10.1016/j.jsbmb.2020.105751] - DOI - PMC - PubMed
Murai 2021 {published data only}
-
- Murai IH, Fernandes AL, Sales LP, Pinto AJ, Giessler KF, Curan CS, et al. Effect of vitamin D 3 supplementation vs placebo on hospital length of stay in patients with severe COVID-19: a multicenter, double-blind, randomized controlled trial (preprint). Medrxiv. [DOI: ]
-
- Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CS, et al. Effect of a single high dose of vitamin D 3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA 2021;Published online February 17, 2021:E1-8. [DOI: 10.1001/jama.2020.26848] - DOI - PMC - PubMed
Rastogi 2020 {published data only}
-
- Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N, et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgraduate Medical Journal 2020;0:1-4. - PubMed
References to studies excluded from this review
Beigmohammadi 2020 {published data only}
-
- Beigmohammadi MT, Bitarafan S, Hoseindokht A, Abdollahi A, Amoozadeh L, Mahmoodi Ali Abadi M, et al. Impact of vitamins A, B, C, D, and E supplementation on improvement and mortality rate in ICU patients with coronavirus-19: a structured summary of a study protocol for a randomized controlled trial. Trials 2020;21(1):614. [PMID: 10.1186/s13063-020-04547-0] - DOI - PMC - PubMed
CTRI/2020/06/026191 {published data only}
-
- CTRI/2020/06/026191. To study the safety and efficacy of vitamin D3,vitamin K2-7 & magnesium, in prevention of COVID 19 infection in health care professional (HCP). www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=45075 2020.
Euctr2020‐001363‐85‐dk {published data only}
-
- Odense University, Hospital. Medication for prevention of Corona-virus for Danish nursing home residents. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_nu....
Euctr2020‐001903‐17 {unpublished data only}
-
- 2020-001903-17. A randomized clinical trial (IIIb) of eficacy of a single dose of Tocilizumab or a combination of Tocilizumab plus vitamin D (single i.m. dose) for the treatment of the COVID-19 hyperimmune complication. Assessment of IL-6. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001903-17/ES.
IRCT20200319046819N1 {published data only}
-
- Beigmohammadi MT, Bitarafan S, Hoseindokht A, Abdollahi A, Amoozadeh L, Mahmoodi Ali Abadi M, et al. Impact of vitamins A, B, C, D, and E supplementation on improvement and mortality rate in ICU patients with coronavirus-19: a structured summary of a study protocol for a randomized controlled trial. Trials 2020;21(1):614. [DOI: 10.1186/s13063-020-04547-0] - DOI - PMC - PubMed
-
- IRCT20200319046819N1. Impact of vitamin B, A, D, E, C supplementation on improvement and mortality rate in patients with COVID-19 admitted in intensive care unit. https://www.irct.ir/trial/46838 (first received April 4, 2020).
IRCT20200705048013N1 {published data only}
-
- Karaj University of Medical Sciences. The effect of Pentoxifylline in patients with Covid-19. http://en.irct.ir/trial/49525.
NCT04351490 {published data only}
-
- NCT04351490. Influence of zinc and vitamin D3 supplementation on survival in elderly institutionalized individuals with CoV-2 SARS infection. https://clinicaltrials.gov/ct2/show/NCT04351490 (first received April 17, 2020).
NCT04395768 {published data only}
-
- National Institute of Integrative Medicine, Australia. Therapies to Prevent Progression of COVID-19, Including Hydroxychloroquine, Azithromycin, Zinc, vitamin D, vitamin B12 with or without vitamin C, a multi-centre, international, randomized trial: the International ALLIANCE Study. https://clinicaltrials.gov/show/NCT04395768.
NCT04399746 {published data only}
-
- NCT04399746. Ivermectin-Azithromycin-Cholecalciferol (IvAzCol) combination therapy for COVID-19. https://clinicaltrials.gov/show/NCT04399746.
NCT04407286 {published data only}
-
- NCT04407286. Vitamin D testing and treatment for COVID 19. https://clinicaltrials.gov/ct2/show/NCT04407286 (First received: May 29, 2020).
NCT04476680 {published data only}
-
- NCT04476680. Reducing asymptomatic infection with vitamin D in coronavirus disease. https://clinicaltrials.gov/ct2/show/NCT04476680 (first received July 20, 2020).
NCT04482686 {published data only}
-
- ProgenaBiome. Trial of combination therapy to treat COVID-19 infection. https://clinicaltrials.gov/show/NCT04482686.
NCT04483635 {published data only}
-
- NCT04483635. Preventing COVID-19 with high-dose vitamin D supplements. https://clinicaltrials.gov/ct2/show/NCT04483635 (first received July 23, 2020).
NCT04507867 {published data only}
-
- NCT04507867. Effect of a Nss to reduce complications in patients with Covid-19 and comorbidities in stage III. https://clinicaltrials.gov/ct2/show/NCT04507867 (first received 11 August 2020).
NCT04535791 {published data only}
-
- NCT04535791. Efficacy of vitamin D supplementation to prevent the risk of acquiring COVID-19 in healthcare workers (COVID-19). https://clinicaltrials.gov/ct2/show/NCT04535791 (first received September 2, 2020).
NCT04565392 {published data only}
-
- drpykessupplementscom. Proof-of-concept randomized placebo-controlled trial of Famotidine for outpatients With COVID-19. https://clinicaltrials.gov/show/NCT04565392.
NCT04579640 {published data only}
-
- NCT04579640. Trial of vitamin D to reduce risk and severity of COVID-19 and other acute respiratory infections (CORONAVIT). https://clinicaltrials.gov/ct2/show/NCT04579640 (first received October 8, 2020).
NCT04596657 {published data only}
-
- NCT04596657. Vitamin D3 supplementation to prevent respiratory tract infections. https://clinicaltrials.gov/ct2/show/NCT04596657 (first received October 22, 2020).
NCT04780061 {published data only}
-
- The Canadian College of Naturopathic, Medicine. Dietary supplements for COVID-19. https://clinicaltrials.gov/show/NCT04780061.
Nogues 2021 {published data only}
-
- Nogues X, Ovejero D, Quesada-Gomez JM, Bouillon R, Arenas D, Pasqual J, et al. Calcifediol treatment and COVID-19-related outcomes (preprint). SSRN. [DOI: ]
References to studies awaiting assessment
IRCT20110726007117N11 {published data only}
-
- IRCT20110726007117N11. Effect of vitamin D supplementation in novel corona virus 2019. https://en.irct.ir/trial/48287 (first received 2020-07-05).
IRCT20200324046850N1 {published data only}
-
- IRCT20200324046850N1. Comparison of vitamin D3 and N-acetylcysteine prescription in COVID-19 patients and their effect on recovery process. https://en.irct.ir/trial/46732 (first received 29 March 2020).
NCT04733625 {published data only}
-
- Kasr El Aini Hospital. TheeEffect of vitamin D tn morbidity and moratlity in patients with SARS-CoV 2 infection. https://clinicaltrials.gov/show/NCT04733625.
References to ongoing studies
CTRI/2020/06/026189 {published data only}
-
- CTRI/2020/06/026189. To compare the safety and efficacy of vitamin D, with magnesium in mild to moderate Covid 19 patients. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=45029 (first received 26/06/2020).
CTRI/2020/12/030083 {published data only}
-
- CTRI/2020/12/030083. To study the role of vitamin D in the treatment of confirmed COVID-19 infection. ctri.nic.in/Clinicaltrials/showallp.php?mid1=46899&EncHid=&userN... (first received 29 December 2020).
EUCTR2020‐001717‐20‐ES {published data only}
-
- EUCTR2020-001717-20-ES. Prevention and treatment with Calcifediol of respiratory problems caused by COVID-19. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_nu... (first received 20 April 2020).
EUCTR2020‐001960‐28‐ES {published data only}
-
- EUCTR2020-001960-28-ES. Efficacy of vitamin D treatment in patients diagnosed with pneumonia who require hospital admission and have vitamin D deficiency and a positive diagnosis for SARS-Cov-2 (COVID-19). https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001960-28/ES (first received 26 Mai 2020).
EUCTR2020‐002274‐28‐ES {published data only}
-
- EUCTR2020-002274-28-ES. Usefulness of vitamin D on morbidity and mortality of SARS-COV-2 virus infection (Covid-19) at the Central University Hospital of Asturias. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-002274-28/ES (first received 2020-05-21).
EUCTRCT2020‐002312‐43‐ES {published data only}
-
- EUCTR2020-002312-43-ES. Clinical trial, randomized, open-label, to evaluate the efficacy of high-dose vitamin D in patients with COVID-19 pneumonia. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-002312-43/ES (first received 2020-05-31).
NCT04334005 {published data only}
-
- NCT04334005. Vitamin D on prevention and treatment of COVID-19. https://clinicaltrials.gov/ct2/show/NCT04334005 (first received April 3, 2020).
NCT04344041 {published data only}
-
- EUCTR2020-001602-34-FR. COvid-19 and Vitamin D supplementation: a multicenter randomized controlled Trial of high dose versus standard dose vitamin D3 in high-risk COVID-19 patients. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001602-34/FR (first received on 3 April 2020).
-
- NCT04344041. COvid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT04344041 (first received 14 April 2020). - PMC - PubMed
NCT04363840 {published data only}
-
- NCT04363840. The LEAD COVID-19 trial: Low-risk, early aspirin and Vitamin D to reduce COVID-19 hospitalizations. https://clinicaltrials.gov/ct2/show/NCT04363840 (first received April 27, 2020).
NCT04385940 {published data only}
-
- NCT04385940. Vitamin D and COVID-19 management. https://clinicaltrials.gov/ct2/show/NCT04385940 (First posted: 13 May 2020).
NCT04386850 {published data only}
-
- NCT04386850. Oral 25-hydroxyvitamin D3 and COVID-19. https://clinicaltrials.gov/ct2/show/NCT04386850 (first received May 13, 2020).
NCT04411446 {published data only}
-
- Mariani J, Tajer C, Antonietti L, Inserra F, Ferder L, Manucha W. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: a structured summary of a study protocol for a randomised controlled trial (CARED-TRIAL). Trials 2021;22(1):111. [DOI: 10.1186/s13063-021-05073-3] - DOI - PMC - PubMed
-
- NCT04411446. Cholecalciferol to improve the outcomes of COVID-19 patients (CARED). https://clinicaltrials.gov/ct2/show/NCT04411446 (First posted June 2, 2020).
NCT04482673 {published data only}
-
- NCT04482673. Vitamin D supplementation in the prevention and mitigation of COVID-19 infection (VitD-COVID19). https://clinicaltrials.gov/ct2/show/NCT04482673 (first received July 22, 2020).
NCT04489628 {published data only}
-
- NCT04489628. Tele-health enabled clinical trial for COVID-19. https://clinicaltrials.gov/ct2/show/NCT04489628 (First posted 28 July 2020).
NCT04502667 {published data only}
-
- NCT04502667. Efficacy of vitamin D treatment in pediatric patients hospitalized by COVID-19 (COVID-19). https://clinicaltrials.gov/ct2/show/NCT04502667 (first received August 6, 2020).
NCT04525820 {published data only}
-
- NCT04525820. High dose vitamin-D substitution in patients with COVID-19: a randomized controlled, multi center study. https://clinicaltrials.gov/ct2/show/NCT04525820 (first received August 25, 2020).
NCT04536298 {published data only}
-
- NCT04536298. Vitamin D and COVID-19 trial (VIVID). https://clinicaltrials.gov/ct2/show/NCT04536298 (First posted 2 September 2020).
NCT04552951 {published data only}
-
- NCT04552951. Effect of vitamin D on morbidity and mortality of the COVID-19 (COVID-VIT-D). https://clinicaltrials.gov/ct2/show/NCT04552951 (first received September 17, 2020).
NCT04621058 {published data only}
-
- NCT04621058. Efficacy of vitamin D treatment in mortality reduction due to COVID-19. https://clinicaltrials.gov/ct2/show/NCT04621058 (first received November 9, 2020).
NCT04636086 {published data only}
-
- NCT04636086. Effect of vitamin D on hospitalized adults with COVID-19 infection. https://clinicaltrials.gov/ct2/show/NCT04636086 (First posted 19 November 2020).
NCT04641195 {published data only}
-
- NCT04641195. Vitamin D and zinc supplementation for improving treatment outcomes among COVID-19 patients in India. https://clinicaltrials.gov/ct2/show/NCT04641195 (first received November 23, 2020).
Additional references
Amrein 2014
-
- Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. Jama 2014;312(15):1520-30. - PubMed
Amrein 2018
Amrein 2020
Barazzoni 2020
Bilezikian 2020
Brandi 2002
-
- Brandi L, Egfjord M, Olgaard K. Pharmacokinetics of 1,25(OH)(2)D(3) and 1alpha(OH)D(3) in normal and uraemic men. Nephrology Dialysis Transplantation 2002;17(5):829-42. - PubMed
Braun 2012
-
- Braun AB, Litonjua AA, Moromizato T, Gibbons FK, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and acute kidney injury in the critically ill. Critical Care Medicine 2012;40(12):3170-9. - PubMed
Buitrago‐Garcia 2020
Cabler 2020
CDC 2020
-
- Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patie... (accessed 22 March 2021).
Chai 2020
-
- Chai KL, Valk SJ, Piechotta V, Kimber C, Monsef I, Doree C, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013600. [DOI: 10.1002/14651858.CD013600.pub3] - DOI - PMC - PubMed
Cochrane LSR
-
- Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode. Available from community.cochrane.org/review-production/production-resources/living-sys... (accessed 22 March 2021).
COMET 2020
-
- Core outcome set developers’ response to COVID-19. Available from www.comet-initiative.org/Studies/Details/1538 (accessed 22 March 2021).
Deeks 2020
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventionsversion 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Eldridge 2016
-
- Eldridge S, Campbell M, Campbell M, Dahota A, Giraudeau B, Higgins JT, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0). Additional considerations for cluster-randomized trials. www.riskofbias.info/welcome/rob-2-0-tool/archive-rob-2-0-cluster-randomi... (accessed 22 March 2021).
EndNote X9 [Computer program]
-
- EndNote X9. The EndNote Team. Clarivate, 2013.
Fraser 2020
-
- Fraser WD, Tang JC, Dutton JJ, Schoenmakers I. Vitamin D measurement, the debates continue, new analytes have emerged, developments have variable outcomes. Calcified Tissue International 2020;106(1):3-13. - PubMed
Grant 2020
Grubaugh 2020
Gupta 2021
Higgins 2003
Higgins 2020a
-
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Higgins 2020b
-
- Higgins JP, Tianging L, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Higgins 2020c
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Higgins 2020d
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Available from: training.cochrane.org/handbook 2020.
Holick 1996
-
- Holick MF. Vitamin D and bone health. Journal of Nutrition 1996;126(4 Suppl):1159s-64s. - PubMed
Holick 2009
Holick 2011
-
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2011;96(7):1911-30. - PubMed
Huang 2020
Hughes 2009
Johns Hopkins University & Medicine
-
- Johns Hopkins University & Medicine Coronavirus Resource Center. Mortality analyses. Available from https://coronavirus.jhu.edu/data/mortality (accessed at 22 March 2021).
Jolliffe 2017
Jones 2008
-
- Jones G. Pharmacokinetics of vitamin D toxicity. American Journal of Clinical Nutrition 2008;88(2):582S-586S. - PubMed
Kreuzberger 2021
Lauer 2020
Leaf 2014
Lee 2009
-
- Lee P, Eisman JA, Center JR . Vitamin D deficiency in critically ill patients. New England Journal of Medicine 2009;360(18):1912-4. - PubMed
Li 2020
-
- Li T, Higgins JP, Deeks JJ. Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Liang 2020
Liu 2006
-
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311(5768):1770-3. - PubMed
Liu 2021
MAGICapp [Computer program]
-
- Norwegian MAGIC Evidence Ecosystem Foundation (powered by UserVoice Inc.) MAGICapp. Brønnøysund (NOR): Norwegian MAGIC Evidence Ecosystem Foundation (powered by UserVoice Inc.), accessed 25 February 2021. Available at magicapp.org.
Malaguarnera 2020
Marshall 2020
Martineau 2017
Martineau 2019
Martucci 2019
-
- Martucci G, Amrein K, Ney J. Vitamin D deficiency in ICU patients. ICU Management & Practice 2019;19(2):114-6.
Microsoft 2018 [Computer program]
-
- Mircosoft Excel. Microsoft Corporation. Microsoft Corporation, 2018. office.microsoft.com/excel.
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. - PubMed
Mokhtari 2020
Munshi 2021
-
- Munshi R, Hussein MH, Toraih EA, Elshazli RM, Jardak C, Sultana N, et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. Journal of Medical Virology 2021;93(2):733-40. - PubMed
Nadim 2020
National COVID‐19 Clinical Evidence Taskforce 2021
-
- National COVID-19 Clinical Evidence Taskforce. Caring for people with COVID-19: supporting Australia’s healthcare professionals with continually updated, evidence-based clinical guidelines. Available from covid19evidence.net.au/#living-guidelines (accessed 22 March 2021).
Ney 2019
-
- Ney J, Heyland DK, Amrein K, Marx G, Grottke O, Choudrakis M, et al. The relevance of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentration for postoperative infections and postoperative organ dysfunctions in cardiac surgery patients: The eVIDenCe study. Clinical Nutrition 2019;38(6):2756-2762. - PubMed
NICE 2020
-
- National Institute for Health and Care Excellence. COVID-19 rapid guideline: vitamin D. Available from https://www.nice.org.uk/guidance/ng187/evidence (assessed 22 March 2021). - PubMed
Nogues 2021
-
- Nogues X, Ovejero D, Quesada-Gomez JM, Bouillon R, Arenas D, Pascual J et al. Calcifediol treatment and COVID-19 related outcomes. Available from https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3771318 (assessed 22 March 2021). - PMC - PubMed
Norman 2014
-
- Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circulation Research 2014;114(2):379-93. - PubMed
Parmar 1998
Pereira 2020
Piechotta 2020
-
- Piechotta V, Valk SJ, Chai KL, Wood EM, Lamikanra A, Kimber C, et al. Safety and effectiveness of convalescent plasma or hyperimmune globulin for people with COVID-19: a rapid review. osf.io/dwf53 2020. [DOI: 10.17605/OSF.IO/DWF53] - DOI - PMC - PubMed
Putzu 2017
-
- Putzu A, Belletti A, Cassina T, Clivio S, Monti G, Zangrillo A, et al. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. Journal of Critical Care 2017;38:109-14. - PubMed
Ramos‐Martinez 2018
-
- Ramos-Martinez E, Lopez-Vancell MR, Fernandez de Cordova-Aguirre JC, Rojas-Serrano J, Chavarria A, Velasco-Medina A, et al. Reduction of respiratory infections in asthma patients supplemented with vitamin D is related to increased serum IL-10 and IFNgamma levels and cathelicidin expression. Cytokine 2018;108:239-46. - PubMed
RevMan Web 2019 [Computer program]
-
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. revman.cochrane.org.
Roth 2020
-
- Roth A, Lütke S, Meinberger D, Hermes G, Sengle G, Koch M, et al. LL-37 fights SARS-CoV-2: The vitamin D-inducible peptide LL-37 inhibits binding of SARS-CoV-2 spike protein to its cellular receptor angiotensin converting enzyme 2 in vitro. bioRxiv 2020/01/01. [DOI: ]
Santesso 2020
Sassi 2018
Schünemann 2020
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Sengupta 2021
Shah 2021
Siemieniuk 2020
-
- Siemieniuk R, Rochwerg B, Agoritsas T, Lamontagne F, Leo Y S, Macdonald H, et al. A living WHO guideline on drugs for Covid-19. BMJ 2020;370: m3379:1-14. [DOI: ] - PubMed
Skoetz 2020
-
- Skoetz N, Goldkuhle M, Van Dalen EC, Akl EA, Trivella M, Mustafa RA, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and evidence profiles. Journal of Clinical Epidemiology 2020;118:124-31. [DOI: 10.1016/j.jclinepi.2019.10.015] - DOI - PubMed
Sterne 2019
Struyf 2020
-
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] - DOI - PMC - PubMed
Tierney 2007
Wang 2020
Wells 2020
WHO 2007
-
- World Health Organization. Cumulative number of reported probable cases of SARS. Available from www.who.int/csr/sars/country/2003_07_11/en (accessed 22 March 2021).
WHO 2019
-
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). Available from www.who.int/emergencies/mers-cov/en (accessed 22 March 2021).
WHO 2020a
-
- World Health Organization. Report of the WHO‐China Joint Mission on coronavirus disease 2019 (COVID‐19). Available from www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-... (accessed 22 March 2021).
WHO 2020b
-
- World Health Organization. Estimating mortality from COVID-19 - scientific brief. Available from www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1 (accessed 22 March 2021).
WHO 2020c
-
- World Health Organization. Corticosteroids for COVID-19 - living guidance 2 September 2020. Available from www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (accessed 22 March 2021).
WHO 2020d
-
- World Health Organization. Draft landscape of COVID-19 candidate vaccines. Available from www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-va... (accessed 22 March 2021).
WHO 2020e
WHO 2020f
-
- World Health Organization (WHO). Clinical management of COVID-19 - interim guidance, 27 May 2020. WHO/2019-nCoV/clinical/2020.4.
WHO 2020g
-
- World Health Organization. COVID-19 Therapeutic Trial Synopsis. Available from www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Tr... (assessed 27 April 2021).
WHO 2021a
-
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. covid19.who.int (accessed 22 March 2021).
WHO2021b
-
- World Health Organization. SARS-CoV-2 Variants. Available from https://www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/ (accessed 22 March 2021).
WHO 2021c
-
- World Health Organization. Weekly epidemiological update - 23 February 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update---... (accessed 22 March 2021).
Williamson 2020
Wu 2017
-
- Wu C, Qiu S, Zhu X, Li L. Vitamin D supplementation and glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Metabolism 2017;73:67-76. - PubMed
Wu 2020
Wu 2020a
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-42. - PubMed
References to other published versions of this review
Stroehlein 2021a
-
- Stroehlein JK, Wallqyist J, Iannizzi C, Benstoem C, Meybohm P, Mikolajewska A, et al. Effects of vitamin D supplementation on clinical outcomes of COVID-19 patients (part of German Evidence Ecosystem CEO-sys). PROSPERO 21 January 2021 (available from https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021232283). [PROSPERO: CRD42021232283]
Stroehlein 2021b
-
- Stroehlein JK, Wallqvist J, Iannizzi C, Mikolajewska A, Metzendorf MI, Benstoem C, et al. Risk of bias assessments for the Cochrane review 'Vitamin D supplementation for the treatment of COVID-19: a living systematic review'. https://zenodo.org/record/4771734#.YKTEFKj7Ryw 2021. [DOI: 10.5281/zenodo.4771734] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

