Light-Mediated Cross-Coupling of Anomeric Trifluoroborates
- PMID: 34029464
- PMCID: PMC9643099
- DOI: 10.1021/acs.orglett.1c01035
Light-Mediated Cross-Coupling of Anomeric Trifluoroborates
Abstract
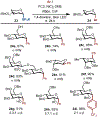
Stereoselective reactions at the anomeric carbon constitute the cornerstone of preparative carbohydrate chemistry. Here, we report stereoselective C-arylation and etherification reactions of anomeric trifluoroborates derived from BMIDA esters. These reactions are characterized by high anomeric selectivities for 2-deoxysugars and broad substrate scope (24 examples), including disaccharides and trifluoroborates with free hydroxyl groups. Taken together, this new class of carbohydrate reagents adds the palette of anomeric nucleophile reagents suitable for efficient installation of C-C bonds.
Figures

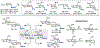


Similar articles
-
Glycosyl Cross-Coupling of Anomeric Nucleophiles: Scope, Mechanism, and Applications in the Synthesis of Aryl C-Glycosides.J Am Chem Soc. 2017 Dec 13;139(49):17908-17922. doi: 10.1021/jacs.7b08707. Epub 2017 Nov 30. J Am Chem Soc. 2017. PMID: 29148749 Free PMC article.
-
Stereoselective entry to beta-linked C-disaccharides using a carbon-Ferrier reaction.Org Lett. 2003 May 15;5(10):1649-52. doi: 10.1021/ol030023t. Org Lett. 2003. PMID: 12735743
-
Palladium(0)-catalyzed cross-coupling of potassium (Z)-2-chloroalk-1-enyl trifluoroborates: a chemo- and stereoselective access to (Z)-chloroolefins and trisubstituted alkenes.Chemistry. 2009 Jun 2;15(23):5793-8. doi: 10.1002/chem.200900425. Chemistry. 2009. PMID: 19396890
-
The use of levoglucosenone and isolevoglucosenone as templates for the construction of C-linked disaccharides.Carbohydr Res. 2006 Jul 24;341(10):1235-52. doi: 10.1016/j.carres.2006.04.008. Epub 2006 May 6. Carbohydr Res. 2006. PMID: 16678805 Review.
-
Venturing beyond Donor-Controlled Glycosylation: New Perspectives toward Anomeric Selectivity.Acc Chem Res. 2018 Mar 20;51(3):628-639. doi: 10.1021/acs.accounts.7b00449. Epub 2018 Feb 22. Acc Chem Res. 2018. PMID: 29469568 Review.
Cited by
-
Exploring the Chemistry and Applications of Thio-, Seleno-, and Tellurosugars.Molecules. 2025 May 5;30(9):2053. doi: 10.3390/molecules30092053. Molecules. 2025. PMID: 40363858 Free PMC article. Review.
-
Desulfurative Borylation of Small Molecules, Peptides, and Proteins.J Am Chem Soc. 2023 Oct 18;145(41):22354-22360. doi: 10.1021/jacs.3c09081. Epub 2023 Oct 9. J Am Chem Soc. 2023. PMID: 37812507 Free PMC article.
-
Diverse Synthesis of C-Glycosides by Stereoselective Ni-Catalyzed Carboboration of Glycals.J Am Chem Soc. 2024 Jul 17;146(28):18866-18872. doi: 10.1021/jacs.4c06246. Epub 2024 Jul 5. J Am Chem Soc. 2024. PMID: 38967118 Free PMC article.
-
Transformations of carbohydrate derivatives enabled by photocatalysis and visible light photochemistry.Chem Sci. 2024 Jan 2;15(4):1204-1236. doi: 10.1039/d3sc05400d. eCollection 2024 Jan 24. Chem Sci. 2024. PMID: 38274059 Free PMC article. Review.
-
C-H Glycosylation of Native Carboxylic Acids: Discovery of Antidiabetic SGLT-2 Inhibitors.ACS Cent Sci. 2023 Jun 9;9(6):1129-1139. doi: 10.1021/acscentsci.3c00201. eCollection 2023 Jun 28. ACS Cent Sci. 2023. PMID: 37396867 Free PMC article.
References
-
- Varki A; Cummings RD; Esko JD; Freeze HH; Stanley P; Bertozzi CR; Hart GW; Etzler ME Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 2009. - PubMed
-
- Imberty A; Varrot A Microbial recognition of human cell surface glycoconjugates. Curr. Opin. Struct. Biol 2008, 18, 567–76. - PubMed
-
- Rudd PM; Elliott T; Cresswell P; Wilson IA; Dwek RA Glycosylation and the immune system. Science 2001, 291, 2370–6. - PubMed
-
- Bililign T; Griffith BR; Thorson JS Structure, activity, synthesis and biosynthesis of aryl-C-glycosides. Nat. Prod. Rep 2005, 22, 742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

