Light-Mediated Cross-Coupling of Anomeric Trifluoroborates
- PMID: 34029464
- PMCID: PMC9643099
- DOI: 10.1021/acs.orglett.1c01035
Light-Mediated Cross-Coupling of Anomeric Trifluoroborates
Abstract
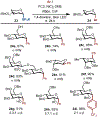
Stereoselective reactions at the anomeric carbon constitute the cornerstone of preparative carbohydrate chemistry. Here, we report stereoselective C-arylation and etherification reactions of anomeric trifluoroborates derived from BMIDA esters. These reactions are characterized by high anomeric selectivities for 2-deoxysugars and broad substrate scope (24 examples), including disaccharides and trifluoroborates with free hydroxyl groups. Taken together, this new class of carbohydrate reagents adds the palette of anomeric nucleophile reagents suitable for efficient installation of C-C bonds.
Figures

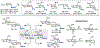


References
-
- Varki A; Cummings RD; Esko JD; Freeze HH; Stanley P; Bertozzi CR; Hart GW; Etzler ME Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 2009. - PubMed
-
- Imberty A; Varrot A Microbial recognition of human cell surface glycoconjugates. Curr. Opin. Struct. Biol 2008, 18, 567–76. - PubMed
-
- Rudd PM; Elliott T; Cresswell P; Wilson IA; Dwek RA Glycosylation and the immune system. Science 2001, 291, 2370–6. - PubMed
-
- Bililign T; Griffith BR; Thorson JS Structure, activity, synthesis and biosynthesis of aryl-C-glycosides. Nat. Prod. Rep 2005, 22, 742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

