AGA Rapid Review and Guideline for SARS-CoV2 Testing and Endoscopy Post-Vaccination: 2021 Update
- PMID: 34029569
- PMCID: PMC8139430
- DOI: 10.1053/j.gastro.2021.05.039
AGA Rapid Review and Guideline for SARS-CoV2 Testing and Endoscopy Post-Vaccination: 2021 Update
Abstract
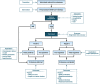
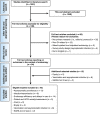
This guideline provides updated recommendations on the role of preprocedure testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) in individuals undergoing endoscopy in the post-vaccination period and replaces the prior guideline from the American Gastroenterological Association (AGA) (released July 29, 2020). Since the start of the pandemic, our increased understanding of transmission has facilitated the implementation of practices to promote patient and health care worker (HCW) safety. Simultaneously, there has been increasing recognition of the potential harm associated with delays in patient care, as well as inefficiency of endoscopy units. With widespread vaccination of HCWs and the general population, a re-evaluation of AGA's prior recommendations was warranted. In order to update the role of preprocedure testing for SARS-CoV2, the AGA guideline panel reviewed the evidence on prevalence of asymptomatic SARS-CoV2 infections in individuals undergoing endoscopy; patient and HCW risk of infections that may be acquired immediately before, during, or after endoscopy; effectiveness of COVID-19 vaccine in reducing risk of infections and transmission; patient and HCW anxiety; patient delays in care and potential impact on cancer burden; and endoscopy volumes. The panel considered the certainty of the evidence, weighed the benefits and harms of routine preprocedure testing, and considered burden, equity, and cost using the Grading of Recommendations Assessment, Development and Evaluation framework. Based on very low certainty evidence, the panel made a conditional recommendation against routine preprocedure testing for SARS-CoV2 in patients scheduled to undergo endoscopy. The panel placed a high value on minimizing additional delays in patient care, acknowledging the reduced endoscopy volumes, downstream impact on delayed cancer diagnoses, and burden of testing on patients.
Keywords: COVID-19; Diagnostic Test; Gastrointestinal Endoscopy; SARS-CoV2.
Copyright © 2021 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Coronavirus disease 2019 (COVID-19): COVID-19 vaccines. US Food and Drug Administration. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise... Accessed May 10, 2021. Available at: - PubMed
-
- Kirzinger A., Kearney A., Hamel L. KFF and Washington Post frontline health care workers survey. The Washington Post/KFF Survey Project. https://www.kff.org/coronavirus-covid-19/poll-finding/kff-washington-pos... Published April 6, 2021. Accessed June 17, 2021. Available at:
-
- Schunemann H.J., Wiercioch W., Brozek J. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101–110. - PubMed
Supplementary References
-
- Hayee B., group S.I.P., Bhandari P. COVID-19 transmission following outpatient endoscopy during pandemic acceleration phase involving SARS-CoV-2 VOC 202012/01 variant in UK. Gut. 2021 - PubMed
-
- Hayee B., Sp group, East J. Multicentre prospective study of COVID-19 transmission following outpatient GI endoscopy in the UK. Gut. 2021;70:825–828. - PubMed
-
- Pena-Rey I., Almazan R., Rodriguez-Camacho E. Resumption of endoscopy in the Galician colorectal cancer screening programme after the COVID-19 lock down: patient safety results. Rev Esp Enferm Dig. 2021;113:119–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

