Effects of altered tau expression on dentate granule cell excitability in mice
- PMID: 34029610
- PMCID: PMC8286323
- DOI: 10.1016/j.expneurol.2021.113766
Effects of altered tau expression on dentate granule cell excitability in mice
Abstract
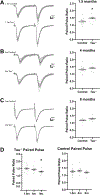
Tauopathies, including Alzheimer's disease, are characterized by progressive accumulation of hyperphosphorylated and pathologic tau protein in association with onset of cognitive and behavioral impairment. Tau pathology is also associated with increased susceptibility to seizures and epilepsy, with tau-/- mice showing seizure resistance in some epilepsy models. To better understand how tau pathology is related to neuronal excitability, we performed whole-cell patch-clamp electrophysiology in dentate gyrus granule cells of tau-/- and human-tau expressing, htau mice. The htau mouse is unique from other transgenic tau models in that the endogenous murine tau gene has been and replaced with readily phosphorylated human tau. We assessed several measures of neuronal excitability, including evoked action potential frequency and excitatory synaptic responses in dentate granule cells from tau-/-, htau, and non-transgenic control mice at 1.5, 4, and 9 months of age. Compared to age matched controls, dentate granule cells from both tau-/- and htau mice had a lower peak frequency of evoked action potentials and greater paired pulse facilitation, suggesting reduced neuronal excitability. Our results suggest that neuronal excitability is more strongly influenced by the absence of functional tau than by the presence of pathologic tau. These results also suggest that tau's effect on neuronal excitability is more complex than previously understood.
Keywords: Dentate granule cell; EPSC; Hippocampus; Microtubule associated protein tau; Tau(−/−); Tauopathy; htau.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Competing interests
The authors have no competing interests to declare.
Figures



Similar articles
-
Altered function of hippocampal CA1 pyramidal neurons in the rTg4510 mouse model of tauopathy.J Alzheimers Dis. 2014;40(2):429-42. doi: 10.3233/JAD-131358. J Alzheimers Dis. 2014. PMID: 24448785
-
Egr1 Expression Is Correlated With Synaptic Activity but Not Intrinsic Membrane Properties in Mouse Adult-Born Dentate Granule Cells.Hippocampus. 2024 Dec;34(12):729-743. doi: 10.1002/hipo.23644. Epub 2024 Oct 15. Hippocampus. 2024. PMID: 39403835
-
Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth.J Neurophysiol. 1999 Apr;81(4):1645-60. doi: 10.1152/jn.1999.81.4.1645. J Neurophysiol. 1999. PMID: 10200201
-
Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology.J Neurosci. 2009 Aug 26;29(34):10741-9. doi: 10.1523/JNEUROSCI.1065-09.2009. J Neurosci. 2009. PMID: 19710325 Free PMC article.
-
Pharmacogenetic neuronal stimulation increases human tau pathology and trans-synaptic spread of tau to distal brain regions in mice.Neurobiol Dis. 2018 Oct;118:161-176. doi: 10.1016/j.nbd.2018.07.003. Epub 2018 Jul 7. Neurobiol Dis. 2018. PMID: 30049665
Cited by
-
Microtubules as Regulators of Neural Network Shape and Function: Focus on Excitability, Plasticity and Memory.Cells. 2022 Mar 8;11(6):923. doi: 10.3390/cells11060923. Cells. 2022. PMID: 35326374 Free PMC article. Review.
-
Alzheimer's disease and epilepsy: An increasingly recognized comorbidity.Front Aging Neurosci. 2022 Nov 10;14:940515. doi: 10.3389/fnagi.2022.940515. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36438002 Free PMC article. Review.
-
Epilepsy and epileptiform activity in late-onset Alzheimer disease: clinical and pathophysiological advances, gaps and conundrums.Nat Rev Neurol. 2024 Mar;20(3):162-182. doi: 10.1038/s41582-024-00932-4. Epub 2024 Feb 14. Nat Rev Neurol. 2024. PMID: 38356056 Review.
-
The Bidirectional Relationship Between Epilepsy and Alzheimer's Disease.Curr Neurol Neurosci Rep. 2025 Feb 8;25(1):18. doi: 10.1007/s11910-025-01404-y. Curr Neurol Neurosci Rep. 2025. PMID: 39921833 Review.
-
Progressive Dysregulation of Tau Phosphorylation in an Animal Model of Temporal Lobe Epilepsy.Neuroscience. 2023 Jul 1;522:42-56. doi: 10.1016/j.neuroscience.2023.04.020. Epub 2023 May 2. Neuroscience. 2023. PMID: 37142182 Free PMC article.
References
-
- Abisambra JF, Blair LJ, Hill SE, Jones JR, Kraft C, Rogers J, Koren J 3rd, Jinwal UK, Lawson L, Johnson AG, Wilcock D, O’Leary JC, Jansen-West K, Muschol M, Golde TE, Weeber EJ, Banko J, Dickey CA, 2010. Phosphorylation dynamics regulate Hsp27-mediated rescue of neuronal plasticity deficits in tau transgenic mice. J Neurosci 30, 15374–15382. - PMC - PubMed
-
- Abisambra JF, Jinwal UK, Blair LJ, O’Leary JC 3rd, Li Q, Brady S, Wang L, Guidi CE, Zhang B, Nordhues BA, Cockman M, Suntharalingham A, Li P, Jin Y, Atkins CA, Dickey CA, 2013. Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation. J Neurosci 33, 9498–9507. - PMC - PubMed
-
- Alcantara-Gonzalez D, Chartampila E, Criscuolo C, Scharfman HE, 2021. Early changes in synaptic and intrinsic properties of dentate gyrus granule cells in a mouse model of Alzheimer’s disease neuropathology and atypical effects of the cholinergic antagonist atropine. Neurobiol Dis, 105274. - PMC - PubMed
-
- Andorfer C, Kress Y, Espinoza M, De Silva R, Tucker KL, Barde Y-A, Duff K, Davies P, 2003. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. Journal of Neurochemistry 86, 582–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

