Tumor resistance to ferroptosis driven by Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and Fatty Acid Biding Protein-4 (FABP4) in tumor microenvironment promote tumor recurrence
- PMID: 34030117
- PMCID: PMC8163990
- DOI: 10.1016/j.redox.2021.102006
Tumor resistance to ferroptosis driven by Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and Fatty Acid Biding Protein-4 (FABP4) in tumor microenvironment promote tumor recurrence
Abstract
Problem: Tumor recurrence is a major clinical issue that represents the principal cause of cancer-related deaths, with few targetable common pathways. Mechanisms by which residual tumors persist and progress under a continuous shift between hypoxia-reoxygenation after neoadjuvent-therapy are unknown. In this study, we investigated the role of lipid metabolism and tumor redox balance in tumor recurrence.
Methods: Lipidomics, proteomics and mass spectrometry imaging approaches where applied to mouse tumor models of recurrence. Genetic and pharmacological inhibitions of lipid mediators in tumors were used in vivo and in functional assays in vitro.
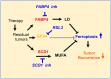
Results: We found that stearoyl-CoA desaturase-1 (SCD1) expressed by cancer cells and fatty acid binding protein-4 (FABP4) produced by tumor endothelial cells (TECs) and adipocytes in the tumor microenvironment (TME) are essential for tumor relapse in response to tyrosine kinase inhibitors (TKI) and chemotherapy. SCD1 and FABP4 were also found upregulated in recurrent human breast cancer samples and correlated with worse prognosis of cancer patients with different types of tumors. Mechanistically, SCD1 leads to fatty acid (FA) desaturation and FABP4 derived from TEM enhances lipid droplet (LD) in cancer cells, which cooperatively protect from oxidative stress-induced ferroptosis. We revealed that lipid mobilization and desaturation elicit tumor intrinsic antioxidant and anti-ferroptotic resources for survival and regrowth in a harsh TME. Inhibition of lipid transport from TME by FABP4 inhibitor reduced tumor regrowth and by genetic - or by pharmacological - targeting SCD1 in vivo, tumor regrowth was abolished completely.
Conclusion: This finding unveils that it is worth taking advantage of tumor lipid addiction, as a tumor vulnerability to design novel treatment strategy to prevent cancer recurrence.
Keywords: Drug-resistance; Hypoxia; Lipid metabolism; ROS-ferroptosis; Reoxygenation; Tumor-microenvironment.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interests.
Figures




References
-
- Harris A.L. Hypoxia--a key regulatory factor in tumour growth. Nat. Rev. Canc. 2002;2:38–47. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop... - PubMed
-
- Reczek C.R., Chandel N.S. The two faces of reactive oxygen species in cancer. Annu. Rev. Cell Biol. 2017;1:79–98. doi: 10.1146/annurev-cancerbio-041916-065808. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

