INSTI-Based Initial Antiretroviral Therapy in Adults with HIV, the HIV Outpatient Study, 2007-2018
- PMID: 34030459
- PMCID: PMC12380107
- DOI: 10.1089/AID.2020.0286
INSTI-Based Initial Antiretroviral Therapy in Adults with HIV, the HIV Outpatient Study, 2007-2018
Abstract
We evaluated treatment duration and viral suppression (VS) outcomes with integrase strand transfer inhibitor (INSTI)-based regimens versus other contemporary regimens among adults in routine HIV care. Eligible participants were seen during January 1, 2007 to June 30, 2018 at nine U.S. HIV clinics, initiated antiretroviral therapy (ART) (baseline date), and had ≥2 clinic visits thereafter. We assessed the probability of remaining on a regimen and achieving HIV RNA <200 copies/mL on initial INSTI versus non-INSTI ART by Kaplan-Meier analyses and their correlates by Cox regression. Among 1,005 patients, 335 (33.3%) were prescribed an INSTI-containing regimen and 670 (66.7%) a non-INSTI regimen, which may have included non-nucleoside reverse transcriptase inhibitors, protease inhibitors, and other agents. In both groups, most patients were male, nonwhite, and aged <50 years. Comparing the INSTI with non-INSTI group, the median baseline log10 HIV viral load (VL; copies/mL) was 4.6 versus 4.5, and the median CD4+ cell count (cells/mm3) was 352 versus 314. In Kaplan-Meier analysis, the estimated probabilities of remaining on initial regimens at 2 and 4 years were 58% and 40% for INSTI and 51% and 33% for non-INSTI group, respectively (log-rank test p = .003). In multivariable models, treatment with an INSTI (vs. non-INSTI) ART was negatively associated with a regimen switch [hazard ratio (HR) 0.67, 95% confidence interval (CI) 0.56-0.81, p < .001] and was positively associated with achieving VS (HR 1.52; CI 1.29-1.79, p < .001), both irrespective of baseline VL levels. Initial INSTI-based regimens were associated with longer treatment durations and better VS than non-INSTI regimens. Results support INSTI regimens as the initial therapy in U.S. treatment guidelines.
Keywords: HIV infection; antiretroviral therapy; integrase strand transfer inhibitors.
Conflict of interest statement
Author Disclosure Statement
No competing financial interests exist.
Figures
References
-
- Lennox JL, DeJesus E, Lazzarin A, et al. : Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: A multicentre, double-blind randomised controlled trial. Lancet 2009;374:796–806. - PubMed
-
- Wohl DA, Cohen C, Gallant JE, et al. : A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: Analysis of week 144 results. J Acquir Immune Defic Syndr 2014;65:e118–e120. - PubMed
-
- Walmsley SL, Antela A, Clumeck N, et al. : Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013;369:1807–1818. - PubMed
-
- Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/what...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

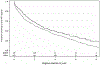
 INSTI;
INSTI; Other. ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitor.
Other. ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitor.