Taxonomic variations in the gut microbiome of gout patients with and without tophi might have a functional impact on urate metabolism
- PMID: 34030623
- PMCID: PMC8142508
- DOI: 10.1186/s10020-021-00311-5
Taxonomic variations in the gut microbiome of gout patients with and without tophi might have a functional impact on urate metabolism
Abstract
Objective: To evaluate the taxonomic composition of the gut microbiome in gout patients with and without tophi formation, and predict bacterial functions that might have an impact on urate metabolism.
Methods: Hypervariable V3-V4 regions of the bacterial 16S rRNA gene from fecal samples of gout patients with and without tophi (n = 33 and n = 25, respectively) were sequenced and compared to fecal samples from 53 healthy controls. We explored predictive functional profiles using bioinformatics in order to identify differences in taxonomy and metabolic pathways.
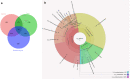
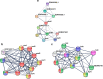
Results: We identified a microbiome characterized by the lowest richness and a higher abundance of Phascolarctobacterium, Bacteroides, Akkermansia, and Ruminococcus_gnavus_group genera in patients with gout without tophi when compared to controls. The Proteobacteria phylum and the Escherichia-Shigella genus were more abundant in patients with tophaceous gout than in controls. Fold change analysis detected nine genera enriched in healthy controls compared to gout groups (Bifidobacterium, Butyricicoccus, Oscillobacter, Ruminococcaceae_UCG_010, Lachnospiraceae_ND2007_group, Haemophilus, Ruminococcus_1, Clostridium_sensu_stricto_1, and Ruminococcaceae_UGC_013). We found that the core microbiota of both gout groups shared Bacteroides caccae, Bacteroides stercoris ATCC 43183, and Bacteroides coprocola DSM 17136. These bacteria might perform functions linked to one-carbon metabolism, nucleotide binding, amino acid biosynthesis, and purine biosynthesis. Finally, we observed differences in key bacterial enzymes involved in urate synthesis, degradation, and elimination.
Conclusion: Our findings revealed that taxonomic variations in the gut microbiome of gout patients with and without tophi might have a functional impact on urate metabolism.
Keywords: Gout; Gut microbiota; Uric acid metabolism.
Conflict of interest statement
The authors declare not to have any conflicts of interest.
Figures




References
-
- Andersen KS, Kirkegaard RH, Karst SM, Albertsen M. ampvis2: an R package to analyse and visualise 16S rRNA amplicon data. bioRxiv; 2018. https://madsalbertsen.github.io/ampvis2/index.html
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

