Estimates of epidemiology, mortality and disease burden associated with progressive fibrosing interstitial lung disease in France (the PROGRESS study)
- PMID: 34030695
- PMCID: PMC8147348
- DOI: 10.1186/s12931-021-01749-1
Estimates of epidemiology, mortality and disease burden associated with progressive fibrosing interstitial lung disease in France (the PROGRESS study)
Abstract
Background: There is a paucity of data on the epidemiology, survival estimates and healthcare resource utilisation and associated costs of patients with progressive fibrosing interstitial lung disease (PF-ILD) in France. An algorithm for extracting claims data was developed to indirectly identify and describe patients with PF-ILD in the French national administrative healthcare database.
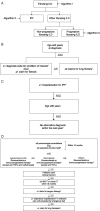
Methods: The French healthcare database, the Système National des Données de Santé (SNDS), includes data related to ambulatory care, hospitalisations and death for 98.8% of the population. In this study, algorithms based on age, diagnosis and healthcare consumption were created to identify adult patients with PF-ILD other than idiopathic pulmonary fibrosis between 2010 and 2017. Incidence, prevalence, survival estimates, clinical features and healthcare resource usage and costs were described among patients with PF-ILD.
Results: We identified a total of 14,413 patients with PF-ILD. Almost half of them (48.1%) were female and the mean (± standard deviation) age was 68.4 (± 15.0) years. Between 2010 and 2017, the estimated incidence of PF-ILD ranged from 4.0 to 4.7/100,000 person-years and the estimated prevalence from 6.6 to 19.4/100,000 persons. The main diagnostic categories represented were exposure-related ILD other than hypersensitivity pneumonitis (n = 3486; 24.2%), idiopathic interstitial pneumonia (n = 3113; 21.6%) and rheumatoid arthritis-associated ILD (n = 2521; 17.5%). Median overall survival using Kaplan-Meier estimation was 3.7 years from the start of progression. During the study, 95.2% of patients had ≥ 1 hospitalisation for respiratory care and 34.3% were hospitalised in an intensive care unit. The median (interquartile range) total specific cost per patient during the follow-up period was €25,613 (10,622-54,287) and the median annual cost per patient was €18,362 (6856-52,026), of which €11,784 (3003-42,097) was related to hospitalisations. Limitations included the retrospective design and identification of cases through an algorithm in the absence of chest high-resolution computed tomography scans and pulmonary function tests.
Conclusions: This large, real-world, longitudinal study provides important insights into the characteristics, epidemiology and healthcare resource utilisation and costs associated with PF-ILD in France using a comprehensive and exhaustive database, and provides vital evidence that PF-ILD represents a high burden on both patients and healthcare services. Trial registration ClinicalTrials.gov, NCT03858842. ISRCTN, ISRCTN12345678. Registered 3 January 2019-Retrospectively registered, https://clinicaltrials.gov/ct2/show/NCT03858842.
Keywords: Algorithms; Epidemiology; Healthcare resource utilisation; Interstitial lung disease; Progressive fibrosis.
Conflict of interest statement
MN reports non-financial support from F. Hoffmann-La Roche, Actelion, AstraZeneca, Chiesi, and Boehringer Ingelheim outside the submitted work. JM reports personal fees from Boehringer Ingelheim during the conduct of the study, research and paid consultancy for Boehringer Ingelheim outside the submitted work, and personal fees from RaDiCo Cohorts (INSERM). DM-B reports personal fees from MaatParma, outside the submitted work. EH reports consulting fees/meeting fees from Actelion, Boehringer Ingelheim, Bayer, GSK, Roche-Chugai, Sanofi-Genzyme; speaking fees from Actelion, GSK, Roche-Chugai; and research funding from Octapharma, CSL Behring, GSK, Roche-Chugai and Actelion. SJ has received fees, funding or reimbursement for national and international conferences, boards, expert or opinion groups, research projects over the past 5 years from Actelion, AIRB, AstraZeneca, Bellorophon Therapeutics, Biogen, BMS, Boehringer Ingelheim, Chiesi, Fibrogen, Galecto Biotech, Genzyme, Gilead, GSK, LVL, Mundipharma, Novartis, Olam Pharm, Pfizer, Pliant Therapeutics, Roche, Sanofi and Savara-Serendex. KLL was an employee of Boehringer Ingelheim France at the time of the research presented in this manuscript. VC reports personal fees and non-financial support from Actelion, Bayer/MSD, and Roche, grants, personal fees, and non-financial support from Boehringer Ingelheim, personal fees from Novartis, Sanofi, Celgene, Galapagos, Galecto and Shionogi, outside the submitted work. SL, LB, SS-M, FB, SM, FTB and LC do not have any disclosures.
Figures



References
-
- Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. - DOI - PMC - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

