Outcomes of valve-in-valve transcatheter aortic valve implantation with and without bioprosthetic valve fracture
- PMID: 34031022
- PMCID: PMC9724846
- DOI: 10.4244/EIJ-D-21-00254
Outcomes of valve-in-valve transcatheter aortic valve implantation with and without bioprosthetic valve fracture
Abstract
Background: Bioprosthetic valve fracture (BVF) is a technique to reduce gradients in valve-in-valve transcatheter aortic valve implantation (VIV-TAVI) procedures. The outcome of VIV-TAVI with BVF has not been compared with VIV-TAVI without BVF.
Aims: The aim of this study was to evaluate the outcome of VIV-TAVI with BVF compared to VIV-TAVI without BVF.
Methods: In total, 81 cases of BVF VIV-TAVI (BVF group) from 14 centres were compared to 79 cases of VIV-TAVI without BVF (control group).
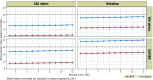
Results: VARC-2-defined device success was 93% in the BVF group and 68.4% in the control group (p<0.001). The mean transvalvular gradient decreased from 37±13 mmHg to 10.8±5.9 mmHg (p<0.001) in the BVF group and from 35±16 mmHg to 15.8±6.8 mmHg (p<0.001) in the control group with a significantly higher final gradient in the control group (p<0.001). The transvalvular gradients did not change significantly over time. In-hospital major adverse events occurred in 3.7% in the BVF group and 7.6% in the control group (p=0.325). A linear mixed model identified BVF, self-expanding transcatheter heart valves (THVs) and other surgical aortic valve (SAV) types other than Mitroflow as predictors of lower transvalvular gradients.
Conclusions: Compared to VIV-TAVI alone, VIV-TAVI with BVF resulted in a significantly lower transvalvular gradient acutely and at follow-up. Independent predictors of lower gradients were the use of self-expanding THVs and the treatment of SAVs other than Mitroflow, irrespective of BVF performance. BVF significantly reduced the gradient independently from transcatheter or surgical valve type.
Conflict of interest statement
M. Abdel-Wahab is a consultant to Medtronic and Boston Scientific. F. Bedogni is a consultant and proctor of Medtronic, Abbott, BSI, and Meril. L. Conradi is a consultant to Edwards, Medtronic, Boston and Abbott. D Hildick-Smith is a proctor/advisor for Abbott, Boston, Edwards, and Medtronic. A. Latib is a consultant for Medtronic, Abbott, Edwards Lifesciences, ICS and Keystone Heart. N.M. Van Mieghem has received research grant support from Abbott Vascular, Boston Scientific, Edwards Lifesciences, Abiomed, Medtronic, PulseCath BV, and Daiichi Sankyon and advisory fees from Abbott, Boston Scientific, Abiomed, Medtronic, PulseCath BV, and Daiichi Sankyo. T. Pilgrim has received research grants to the institution from Boston Scientific, Edwards Lifesciences and Biotronik; speaker fees from Boston Scientific and Biotronik, declares consultancy for HighLifeSAS (CEC), and proctoring for Medtronic. M. Taramasso is a consultant for Abbott, Boston Scientific, 4tech, and CoreMedic, and has received honoraria from Edwards Lifesciences, Mitraltech, and SwissVortex. L. Testa is a medical proctor for Boston Scientific, Meril, Concept Medical, Abbott, and Philips, and is an advisory board member and/or has received speaker fees and/or institutional research grants from BSCI, Philips, Abbott, Medtronic, Terumo, and Concept Medical. J. Webb is a consultant to and receives research funding from Edwards Lifesciences, Abbott, and Boston Scientific. S. Windecker reports research and educational grants to the institution from Abbott, Amgen, AstraZeneca, BMS, Bayer, Biotronik, Boston Scientific, Cardinal Health, Cardiovalve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, Infraredx, Johnson & Johnson, Medicure, Medtronic, Novartis, Polares, OrPha Suisse, Pfizer, Regeneron, Sanofi-Aventis, Sinomed, Terumo, and V-Wave. S. Windecker also serves as unpaid advisory board member and/or unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Sinomed, V-Wave and Xeltis, but has not received personal payments by pharmaceutical companies or device manufacturers. He is also a member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration. S. Windecker is an unpaid member of the Pfizer Research Award selection committee in Switzerland and of the Women as One Awards Committee. He is a member of the Clinical Study Group of the Deutsches Zentrum für Herz Kreislauf-Forschung and of the Advisory Board of the Australian Victorian Heart Institute. He is chairperson of the ESC Congress Program Committee and Deputy Editor of JACC CV Interventions. J. Schofer is a consultant for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Figures




References
-
- Dvir D, Webb JG, Bleiziffer S, Pasic M, Waksman R, Kodali S, Barbanti M, Latib A, Schaefer U, Rodés-Cabau J, Treede H, Piazza N, Hildick-Smith D, Himbert D, Walther T, Hengstenberg C, Nissen H, Bekeredjian R, Presbitero P, Ferrari E, Segev A, de Weger A, Windecker S, Moat NE, Napodano M, Wilbring M, Cerillo AG, Brecker S, Tchetche D, Lefevre T, De Marco F, Fiorina C, Petronio AS, Teles RC, Testa L, Laborde JC, Leon MB, Kornowski R Valve-in-Valve International Data Registry Investigators. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162–70. - PubMed
-
- Head SJ, Mokhles MM, Osnabrugge RL, Pibarot P, Mack MJ, Takkenberg JJ, Bogers AJ, Kappetein AP. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur Heart J. 2012;33:1518–29. doi: 10.1093/eurheartj/ehs003. - DOI - PubMed
-
- Webb JG, Mack MJ, White JM, Dvir D, Blanke P, Herrmann HC, Leipsic J, Kodali SK, Makkar R, Miller DC, Pibarot P, Pichard A, Satler LF, Svensson L, Alu MC, Suri RM, Leon MB. Transcatheter Aortic Valve Implantation Within Degenerated Aortic Surgical Bioprostheses: PARTNER 2 Valve-in-Valve Registry. J Am Coll Cardiol. 2017;69:2253–62. doi: 10.1016/j.jacc.2017.02.057. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

