Early Humoral Responses of Hemodialysis Patients after COVID-19 Vaccination with BNT162b2
- PMID: 34031181
- PMCID: PMC8425619
- DOI: 10.2215/CJN.03700321
Early Humoral Responses of Hemodialysis Patients after COVID-19 Vaccination with BNT162b2
Abstract
Background and objectives: Patients receiving hemodialysis are at high risk for both severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe coronavirus disease 2019. A lifesaving vaccine is available, but sensitivity to vaccines is generally lower in patients on dialysis. Little is yet known about antibody responses after coronavirus disease 2019 (COVID-19) vaccination in this vulnerable group.
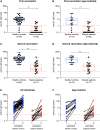
Design, setting, participants, and measurements: In this prospective single-center study, we included 22 patients on dialysis and 46 healthy controls from Heidelberg University Hospital between December 2020 and February 2021. We measured anti-S1 IgG with a threshold index for detection greater than one, neutralizing antibodies with a threshold for viral neutralization of ≥30%, and antibodies against different SARS-CoV2 fragments 17-22 days after the first dose and 18-22 days after the second dose of the mRNA vaccine BNT162b2.
Results: After the first vaccine dose, four of 22 (18%) patients on dialysis compared with 43 of 46 (93%) healthy controls developed positive anti-S1 IgG, with a median anti-S1 IgG index of 0.2 (interquartile range, 0.1-0.7) compared with nine (interquartile range, 4-16), respectively. SARS-CoV2 neutralizing antibodies exceeded the threshold for neutralization in four of 22 (18%) patients on dialysis compared with 43 of 46 (93%) healthy controls, with a median percent inhibition of 11 (interquartile range, 3-24) compared with 65 (interquartile range, 49-75), respectively. After the second dose, 14 of 17 (82%) patients on dialysis developed neutralizing antibodies exceeding the threshold for viral neutralization and antibodies against the receptor binding S1 domain of the spike protein, compared with 46 of 46 (100%) healthy controls, respectively. The median percent inhibition was 51 (interquartile range, 32-86) compared with 98 (interquartile range, 97-98) in healthy controls.
Conclusions: Patients receiving long-term hemodialysis show a reduced antibody response to the first and second doses of the mRNA vaccine BNT162b2. The majority (82%) develop neutralizing antibodies after the second dose but at lower levels than healthy controls.
Keywords: COVID-19; SARS-CoV-2; dialysis; hemodialysis; humoral response; vaccination.
Copyright © 2021 by the American Society of Nephrology.
Figures




Comment in
-
mRNA COVID-19 Vaccine for People with Kidney Failure: Hope but Prudence Warranted.Clin J Am Soc Nephrol. 2021 Jul;16(7):996-998. doi: 10.2215/CJN.04500421. Epub 2021 May 27. Clin J Am Soc Nephrol. 2021. PMID: 34045299 Free PMC article. No abstract available.
References
-
- Couchoud C, Bayer F, Ayav C, Béchade C, Brunet P, Chantrel F, Frimat L, Galland R, Hourmant M, Laurain E, Lobbedez T, Mercadal L, Moranne O; French REIN registry: Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int 98: 1519–1529, 2020 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

