Zika virus employs the host antiviral RNase L protein to support replication factory assembly
- PMID: 34031250
- PMCID: PMC8179202
- DOI: 10.1073/pnas.2101713118
Zika virus employs the host antiviral RNase L protein to support replication factory assembly
Abstract
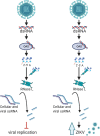
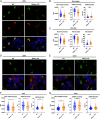
Infection with the flavivirus Zika virus (ZIKV) can result in tissue tropism, disease outcome, and route of transmission distinct from those of other flaviviruses; therefore, we aimed to identify host machinery that exclusively promotes the ZIKV replication cycle, which can inform on differences at the organismal level. We previously reported that deletion of the host antiviral ribonuclease L (RNase L) protein decreases ZIKV production. Canonical RNase L catalytic activity typically restricts viral infection, including that of the flavivirus dengue virus (DENV), suggesting an unconventional, proviral RNase L function during ZIKV infection. In this study, we reveal that an inactive form of RNase L supports assembly of ZIKV replication factories (RFs) to enhance infectious virus production. Compared with the densely concentrated ZIKV RFs generated with RNase L present, deletion of RNase L induced broader subcellular distribution of ZIKV replication intermediate double-stranded RNA (dsRNA) and NS3 protease, two constituents of ZIKV RFs. An inactive form of RNase L was sufficient to contain ZIKV genome and dsRNA within a smaller RF area, which subsequently increased infectious ZIKV release from the cell. Inactive RNase L can interact with cytoskeleton, and flaviviruses remodel cytoskeleton to construct RFs. Thus, we used the microtubule-stabilization drug paclitaxel to demonstrate that ZIKV repurposes RNase L to facilitate the cytoskeleton rearrangements required for proper generation of RFs. During infection with flaviviruses DENV or West Nile Kunjin virus, inactive RNase L did not improve virus production, suggesting that a proviral RNase L role is not a general feature of all flavivirus infections.
Keywords: OAS3; RNase L; Zika virus; flavivirus; replication factories.
Conflict of interest statement
Competing interest statement: S.R.W. is on the scientific advisory board of Immunome, Inc. and Ocugen, Inc. R.H.S. is a consultant to Inception Therapeutics, Inc.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

