The metabolic hormone leptin promotes the function of TFH cells and supports vaccine responses
- PMID: 34031386
- PMCID: PMC8144586
- DOI: 10.1038/s41467-021-23220-x
The metabolic hormone leptin promotes the function of TFH cells and supports vaccine responses
Abstract
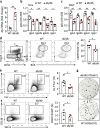
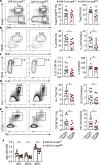
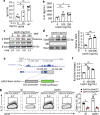
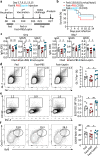
Follicular helper T (TFH) cells control antibody responses by supporting antibody affinity maturation and memory formation. Inadequate TFH function has been found in individuals with ineffective responses to vaccines, but the mechanism underlying TFH regulation in vaccination is not understood. Here, we report that lower serum levels of the metabolic hormone leptin associate with reduced vaccine responses to influenza or hepatitis B virus vaccines in healthy populations. Leptin promotes mouse and human TFH differentiation and IL-21 production via STAT3 and mTOR pathways. Leptin receptor deficiency impairs TFH generation and antibody responses in immunisation and infection. Similarly, leptin deficiency induced by fasting reduces influenza vaccination-mediated protection for the subsequent infection challenge, which is mostly rescued by leptin replacement. Our results identify leptin as a regulator of TFH cell differentiation and function and indicate low levels of leptin as a risk factor for vaccine failure.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Altered proportions of circulating CXCR5+ helper T cells do not dampen influenza vaccine responses in children with rheumatic disease.Vaccine. 2019 Jun 19;37(28):3685-3693. doi: 10.1016/j.vaccine.2019.05.037. Epub 2019 May 22. Vaccine. 2019. PMID: 31126860
-
Strong influenza-induced TFH generation requires CD4 effectors to recognize antigen locally and receive signals from continuing infection.Proc Natl Acad Sci U S A. 2022 Feb 22;119(8):e2111064119. doi: 10.1073/pnas.2111064119. Proc Natl Acad Sci U S A. 2022. PMID: 35177472 Free PMC article.
-
A critical role of T follicular helper cells in human mucosal anti-influenza response that can be enhanced by immunological adjuvant CpG-DNA.Antiviral Res. 2016 Aug;132:122-30. doi: 10.1016/j.antiviral.2016.05.021. Epub 2016 May 28. Antiviral Res. 2016. PMID: 27247060
-
Breadth of Antibody Responses during Influenza Virus Infection and Vaccination.Trends Immunol. 2020 May;41(5):394-405. doi: 10.1016/j.it.2020.03.005. Epub 2020 Apr 4. Trends Immunol. 2020. PMID: 32265127 Review.
-
Role of TRAFs in Signaling Pathways Controlling T Follicular Helper Cell Differentiation and T Cell-Dependent Antibody Responses.Front Immunol. 2018 Oct 22;9:2412. doi: 10.3389/fimmu.2018.02412. eCollection 2018. Front Immunol. 2018. PMID: 30405612 Free PMC article. Review.
Cited by
-
T Cell/B Cell Interactions in the Establishment of Protective Immunity.Vaccines (Basel). 2021 Sep 24;9(10):1074. doi: 10.3390/vaccines9101074. Vaccines (Basel). 2021. PMID: 34696182 Free PMC article. Review.
-
Dimethyl fumarate ameliorates chronic graft-versus-host disease by inhibiting Tfh differentiation via Nrf2.Leukemia. 2025 Feb;39(2):473-481. doi: 10.1038/s41375-024-02475-5. Epub 2024 Nov 23. Leukemia. 2025. PMID: 39580582
-
Obesity Dysregulates the Immune Response to Influenza Infection and Vaccination Through Metabolic and Inflammatory Mechanisms.Annu Rev Nutr. 2022 Aug 22;42:67-89. doi: 10.1146/annurev-nutr-062320-115937. Annu Rev Nutr. 2022. PMID: 35995048 Free PMC article. Review.
-
Emerging mechanisms of obesity-associated immune dysfunction.Nat Rev Endocrinol. 2024 Mar;20(3):136-148. doi: 10.1038/s41574-023-00932-2. Epub 2023 Dec 21. Nat Rev Endocrinol. 2024. PMID: 38129700 Review.
-
Systemic immunometabolism and responses to vaccines: insights from T and B cell perspectives.Int Immunol. 2023 Dec 23;35(12):571-582. doi: 10.1093/intimm/dxad021. Int Immunol. 2023. PMID: 37330692 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

