Metabolic plasticity enables lifestyle transitions of Porphyromonas gingivalis
- PMID: 34031416
- PMCID: PMC8144566
- DOI: 10.1038/s41522-021-00217-4
Metabolic plasticity enables lifestyle transitions of Porphyromonas gingivalis
Abstract
Our understanding of how the oral anaerobe Porphyromonas gingivalis can persist below the gum line, induce ecological changes, and promote polymicrobial infections remains limited. P. gingivalis has long been described as a highly proteolytic and asaccharolytic pathogen that utilizes protein substrates as the main source for energy production and proliferation. Here, we report that P. gingivalis displays a metabolic plasticity that enables the exploitation of non-proteinaceous substrates, specifically the monocarboxylates pyruvate and lactate, as well as human serum components, for colonization and biofilm formation. We show that anabolism of carbohydrates from pyruvate is powered by catabolism of amino acids. Concomitantly, the expression of fimbrial adhesion is upregulated, leading to the enhancement of biofilm formation, stimulation of multispecies biofilm development, and increase of colonization and invasion of the primary gingival epithelial cells by P. gingivalis. These studies provide the first glimpse into the metabolic plasticity of P. gingivalis and its adaptation to the nutritional condition of the host niche. Our findings support the model that in response to specific nutritional parameters, P. gingivalis has the potential to promote host colonization and development of a pathogenic community.
Conflict of interest statement
The authors declare no competing interests.
Figures


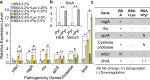



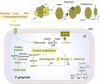
Similar articles
-
Community Development between Porphyromonas gingivalis and Candida albicans Mediated by InlJ and Als3.mBio. 2018 Apr 24;9(2):e00202-18. doi: 10.1128/mBio.00202-18. mBio. 2018. PMID: 29691333 Free PMC article.
-
Dual lifestyle of Porphyromonas gingivalis in biofilm and gingival cells.Microb Pathog. 2016 May;94:42-7. doi: 10.1016/j.micpath.2015.10.003. Epub 2015 Oct 9. Microb Pathog. 2016. PMID: 26456558 Review.
-
Role of the Streptococcus gordonii SspB protein in the development of Porphyromonas gingivalis biofilms on streptococcal substrates.Microbiology (Reading). 2002 Jun;148(Pt 6):1627-1636. doi: 10.1099/00221287-148-6-1627. Microbiology (Reading). 2002. PMID: 12055284
-
Biofilm formation by Porphyromonas gingivalis and Streptococcus gordonii.J Periodontal Res. 1998 Aug;33(6):323-7. doi: 10.1111/j.1600-0765.1998.tb02206.x. J Periodontal Res. 1998. PMID: 9777582
-
Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis.Microbiol Mol Biol Rev. 1998 Dec;62(4):1244-63. doi: 10.1128/MMBR.62.4.1244-1263.1998. Microbiol Mol Biol Rev. 1998. PMID: 9841671 Free PMC article. Review.
Cited by
-
Functional Degeneracy in Paracoccus denitrificans Pd1222 Is Coordinated via RamB, Which Links Expression of the Glyoxylate Cycle to Activity of the Ethylmalonyl-CoA Pathway.Appl Environ Microbiol. 2023 Jul 26;89(7):e0023823. doi: 10.1128/aem.00238-23. Epub 2023 Jun 15. Appl Environ Microbiol. 2023. PMID: 37318336 Free PMC article.
-
Cortisol Promotes Surface Translocation of Porphyromonas gingivalis.Pathogens. 2022 Aug 27;11(9):982. doi: 10.3390/pathogens11090982. Pathogens. 2022. PMID: 36145414 Free PMC article.
-
Growth of Porphyromonas gingivalis on human serum albumin triggers programmed cell death.J Oral Microbiol. 2022 Dec 22;15(1):2161182. doi: 10.1080/20002297.2022.2161182. eCollection 2023. J Oral Microbiol. 2022. PMID: 36570975 Free PMC article.
-
Genomic and phenotypic comparison of Prevotella intermedia strains possessing different virulence in vivo.Virulence. 2022 Dec;13(1):1133-1145. doi: 10.1080/21505594.2022.2095718. Virulence. 2022. PMID: 35791444 Free PMC article.
-
Atypical cyclic di-AMP signaling is essential for Porphyromonas gingivalis growth and regulation of cell envelope homeostasis and virulence.NPJ Biofilms Microbiomes. 2022 Jul 6;8(1):53. doi: 10.1038/s41522-022-00316-w. NPJ Biofilms Microbiomes. 2022. PMID: 35794154 Free PMC article.
References
-
- Maddi A, Scannapieco FA. Oral biofilms, oral and periodontal infections, and systemic disease. Am. J. Dent. 2013;26:249–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

