Molecular model of a sensor of two-component signaling system
- PMID: 34031444
- PMCID: PMC8144572
- DOI: 10.1038/s41598-021-89613-6
Molecular model of a sensor of two-component signaling system
Abstract
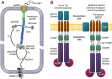
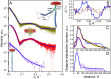
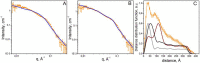
Two-component systems (TCS) are widespread signaling systems present in all domains of life. TCS typically consist of a signal receptor/transducer and a response regulator. The receptors (histidine kinases, chemoreceptors and photoreceptors) are often embedded in the membrane and have a similar modular structure. Chemoreceptors were shown to function in highly ordered arrays, with trimers of dimers being the smallest functional unit. However, much less is known about photoreceptors. Here, we use small-angle scattering (SAS) to show that detergent-solubilized sensory rhodopsin II in complex with its cognate transducer forms dimers at low salt concentration, which associate into trimers of dimers at higher buffer molarities. We then fit an atomistic model of the whole complex into the SAS data. The obtained results suggest that the trimer of dimers is "tripod"-shaped and that the contacts between the dimers occur only through their cytoplasmic regions, whereas the transmembrane regions remain unconnected.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

