Predicting MHC I restricted T cell epitopes in mice with NAP-CNB, a novel online tool
- PMID: 34031450
- PMCID: PMC8144223
- DOI: 10.1038/s41598-021-89927-5
Predicting MHC I restricted T cell epitopes in mice with NAP-CNB, a novel online tool
Abstract
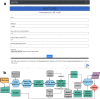
Lack of a dedicated integrated pipeline for neoantigen discovery in mice hinders cancer immunotherapy research. Novel sequential approaches through recurrent neural networks can improve the accuracy of T-cell epitope binding affinity predictions in mice, and a simplified variant selection process can reduce operational requirements. We have developed a web server tool (NAP-CNB) for a full and automatic pipeline based on recurrent neural networks, to predict putative neoantigens from tumoral RNA sequencing reads. The developed software can estimate H-2 peptide ligands, with an AUC comparable or superior to state-of-the-art methods, directly from tumor samples. As a proof-of-concept, we used the B16 melanoma model to test the system's predictive capabilities, and we report its putative neoantigens. NAP-CNB web server is freely available at http://biocomp.cnb.csic.es/NeoantigensApp/ with scripts and datasets accessible through the download section.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

