Power determination in vitamin D randomised control trials and characterising factors affecting it through a novel simulation-based tool
- PMID: 34031451
- PMCID: PMC8144427
- DOI: 10.1038/s41598-021-90019-7
Power determination in vitamin D randomised control trials and characterising factors affecting it through a novel simulation-based tool
Erratum in
-
Publisher Correction: Power determination in vitamin D randomised control trials and characterising factors affecting it through a novel simulation-based tool.Sci Rep. 2021 Jun 22;11(1):13387. doi: 10.1038/s41598-021-92486-4. Sci Rep. 2021. PMID: 34158550 Free PMC article. No abstract available.
Abstract
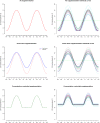
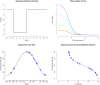
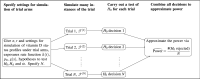
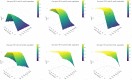
Thousands of observational studies have linked vitamin D deficiency with numerous diseases, but randomised controlled trials (RCTs) often fail to show benefit of supplementation. Population characteristics and trial design have long been suspected to undermine power but were not systematically investigated. We propose a flexible generative model to characterise benefit of vitamin D supplementation at the individual level, and use this to quantify power in RCTs. The model can account for seasonality and population heterogeneity. In a simulated 1-year trial with 1000 participants per arm and assuming a 25-hydroxyvitamin D (25OHD) increase of 20 nmol/L due to the intervention, with baseline 25OHD in the population of 15, 35, 50, 60 and 75 nmol/L, the power to detect intervention effect was 77%, 99%, 95%, 68% and 19%, respectively. The number of participants required per arm to achieve 80% power according to baseline 25OHD of 15-60 nmol/L was 1200, 400, 600 and 1400, respectively. As expected, larger increases in 25OHD due to supplementation improved power in certain scenarios. For a population baseline of 50 nmol/L, with 1500 participants in each arm, there was 100% power to detect a 20 nmol/L 25OHD increase while it was 76% for a 10 nmol/L increase. Population characteristics and trial design, including temporal considerations, have a dramatic impact on power and required sample size in vitamin D RCTs.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Entrenas Castillo M, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

