Folliculin haploinsufficiency causes cellular dysfunction of pleural mesothelial cells
- PMID: 34031471
- PMCID: PMC8144428
- DOI: 10.1038/s41598-021-90184-9
Folliculin haploinsufficiency causes cellular dysfunction of pleural mesothelial cells
Abstract
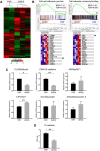
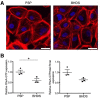
Birt-Hogg-Dubé syndrome (BHDS), an autosomal dominant inheritance disease caused by folliculin (FLCN) mutations, is associated with lung cysts and spontaneous pneumothorax. The possibility of FLCN haploinsufficiency in pleural mesothelial cells (PMCs) contributing to development of pneumothorax has not yet been clarified. Electron microscopy revealed exposed intercellular boundaries between PMCs on visceral pleura and decreased electron density around the adherens junctions in BHDS. To characterize cellular function of PMCs in BHDS patients (BHDS-PMCs), during surgery for pneumothorax, we established the flow cytometry-based methods of isolating high-purity PMCs from pleural lavage fluid. BHDS-PMCs showed impaired cell attachment and a significant decrease in proliferation and migration, but a significant increase in apoptosis compared with PMCs from primary spontaneous pneumothorax (PSP) patients (PSP-PMCs). Microarray analysis using isolated PMCs revealed a significant alteration in the expression of genes belonging to Gene Ontology terms "cell-cell adhesion junction" and "cell adhesion molecule binding". Gene set enrichment analysis demonstrated that CDH1, encoding E-cadherin, was identified in the down-regulated leading edge of a plot in BHDS-PMCs. AMPK and LKB1 activation were significantly impaired in BHDS-PMCs compared with PSP-PMCs. Our findings indicate that FLCN haploinsufficiency may affect the E-cadherin-LKB1-AMPK axis and lead to abnormal cellular function in BHDS-PMCs.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Haploinsufficiency of the folliculin gene leads to impaired functions of lung fibroblasts in patients with Birt-Hogg-Dubé syndrome.Physiol Rep. 2016 Nov;4(21):e13025. doi: 10.14814/phy2.13025. Epub 2016 Nov 15. Physiol Rep. 2016. PMID: 27905298 Free PMC article.
-
FLCN-regulated miRNAs suppressed reparative response in cells and pulmonary lesions of Birt-Hogg-Dubé syndrome.Thorax. 2020 Jun;75(6):476-485. doi: 10.1136/thoraxjnl-2019-213225. Epub 2020 Mar 17. Thorax. 2020. PMID: 32184379 Free PMC article.
-
The Relevance of Family History Taking in the Detection and Management of Birt-Hogg-Dubé Syndrome.Respiration. 2019;98(2):125-132. doi: 10.1159/000498973. Epub 2019 Jul 2. Respiration. 2019. PMID: 31266032
-
Birt-Hogg-Dubé syndrome. State-of-the-art review with emphasis on pulmonary involvement.Respir Med. 2015 Mar;109(3):289-96. doi: 10.1016/j.rmed.2014.11.008. Epub 2014 Dec 9. Respir Med. 2015. PMID: 25519092 Review.
-
Birt-Hogg-Dubé syndrome: Clinical and molecular aspects of recently identified kidney cancer syndrome.Int J Urol. 2016 Mar;23(3):204-10. doi: 10.1111/iju.13015. Epub 2015 Nov 25. Int J Urol. 2016. PMID: 26608100 Review.
Cited by
-
Clinical and genetic features of 334 Asian patients with Birt-Hogg-Dubé syndrome (BHDS) who presented with pulmonary cysts with or without a history of pneumothorax, with special reference to BHDS-associated pneumothorax.PLoS One. 2023 Jul 25;18(7):e0289175. doi: 10.1371/journal.pone.0289175. eCollection 2023. PLoS One. 2023. PMID: 37490463 Free PMC article. Review.
-
Novel 3D organotypic co-culture model of pleura.PLoS One. 2022 Dec 1;17(12):e0276978. doi: 10.1371/journal.pone.0276978. eCollection 2022. PLoS One. 2022. PMID: 36454800 Free PMC article.
-
Genetic insight into Birt-Hogg-Dubé syndrome in Indian patients reveals novel mutations at FLCN.Orphanet J Rare Dis. 2022 Apr 27;17(1):176. doi: 10.1186/s13023-022-02326-5. Orphanet J Rare Dis. 2022. PMID: 35477461 Free PMC article.
-
Thyroid Carcinoma in Birt-Hogg-Dubé Syndrome: Case Series and Review of Literature.Thyroid. 2025 Jul;35(7):828-835. doi: 10.1089/thy.2024.0641. Epub 2025 Jun 5. Thyroid. 2025. PMID: 40658617 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

