The limit of tolerable micromotion for implant osseointegration: a systematic review
- PMID: 34031476
- PMCID: PMC8144379
- DOI: 10.1038/s41598-021-90142-5
The limit of tolerable micromotion for implant osseointegration: a systematic review
Abstract
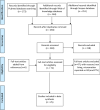
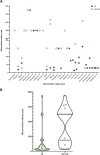
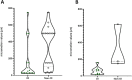
Much research effort is being invested into the development of porous biomaterials that enhance implant osseointegration. Large micromotions at the bone-implant interface impair this osseointegration process, resulting in fibrous capsule formation and implant loosening. This systematic review compiled all the in vivo evidence available to establish if there is a universal limit of tolerable micromotion for implant osseointegration. The protocol was registered with the International Prospective Register for Systematic Reviews (ID: CRD42020196686). Pubmed, Scopus and Web of Knowledge databases were searched for studies containing terms relating to micromotion and osseointegration. The mean value of micromotion for implants that osseointegrated was 32% of the mean value for those that did not (112 ± 176 µm versus 349 ± 231 µm, p < 0.001). However, there was a large overlap in the data ranges with no universal limit apparent. Rather, many factors were found to combine to affect the overall outcome including loading time, the type of implant and the material being used. The tables provided in this review summarise these factors and will aid investigators in identifying the most relevant micromotion values for their biomaterial and implant development research.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Role of implants surface modification in osseointegration: A systematic review.J Biomed Mater Res A. 2020 Mar;108(3):470-484. doi: 10.1002/jbm.a.36829. Epub 2019 Nov 14. J Biomed Mater Res A. 2020. PMID: 31664764
-
The effect of implant thread design on stress distribution in anisotropic bone with different osseointegration conditions: a finite element analysis.Int J Oral Maxillofac Implants. 2015 Nov-Dec;30(6):1317-26. doi: 10.11607/jomi.4091. Epub 2015 Oct 16. Int J Oral Maxillofac Implants. 2015. PMID: 26478976
-
Effect of porous orthopaedic implant material and structure on load sharing with simulated bone ingrowth: A finite element analysis comparing titanium and PEEK.J Mech Behav Biomed Mater. 2018 Apr;80:68-76. doi: 10.1016/j.jmbbm.2018.01.017. J Mech Behav Biomed Mater. 2018. PMID: 29414477 Free PMC article.
-
Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK.Biomaterials. 2018 Dec;185:106-116. doi: 10.1016/j.biomaterials.2018.09.009. Epub 2018 Sep 13. Biomaterials. 2018. PMID: 30236838
-
Implants in bone: part II. Research on implant osseointegration: material testing, mechanical testing, imaging and histoanalytical methods.Oral Maxillofac Surg. 2014 Dec;18(4):355-72. doi: 10.1007/s10006-013-0397-2. Epub 2013 Feb 21. Oral Maxillofac Surg. 2014. PMID: 23430020 Review.
Cited by
-
Evaluation of Cortical Bone Thickness of Posterior Implant Sites Using CBCT in Iraqi Population.Int J Dent. 2022 Sep 5;2022:5723397. doi: 10.1155/2022/5723397. eCollection 2022. Int J Dent. 2022. PMID: 36105382 Free PMC article.
-
Cementless fixation in total joint arthroplasty: Factors impacting osseointegration.J Clin Orthop Trauma. 2024 Dec 13;61:102871. doi: 10.1016/j.jcot.2024.102871. eCollection 2025 Feb. J Clin Orthop Trauma. 2024. PMID: 39816715 Review.
-
In vitro and in silico methods for the biomechanical assessment of osseointegrated transfemoral prostheses: a systematic review.Front Bioeng Biotechnol. 2023 Aug 17;11:1237919. doi: 10.3389/fbioe.2023.1237919. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37662439 Free PMC article. Review.
-
Stemless reverse shoulder arthroplasty neck shaft angle influences humeral component time-zero fixation and survivorship: a cadaveric biomechanical assessment.JSES Int. 2024 Apr 19;8(4):880-887. doi: 10.1016/j.jseint.2024.04.001. eCollection 2024 Jul. JSES Int. 2024. PMID: 39035638 Free PMC article.
-
Micromotion and stress shielding between taper fluted and cylindrical femoral stems for Paprosky bone deficiency type IIIB.Musculoskelet Surg. 2023 Dec;107(4):391-396. doi: 10.1007/s12306-023-00781-2. Epub 2023 Mar 21. Musculoskelet Surg. 2023. PMID: 36944751
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

