Patients with COVID-19: in the dark-NETs of neutrophils
- PMID: 34031543
- PMCID: PMC8142290
- DOI: 10.1038/s41418-021-00805-z
Patients with COVID-19: in the dark-NETs of neutrophils
Abstract
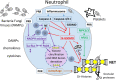
SARS-CoV-2 infection poses a major threat to the lungs and multiple other organs, occasionally causing death. Until effective vaccines are developed to curb the pandemic, it is paramount to define the mechanisms and develop protective therapies to prevent organ dysfunction in patients with COVID-19. Individuals that develop severe manifestations have signs of dysregulated innate and adaptive immune responses. Emerging evidence implicates neutrophils and the disbalance between neutrophil extracellular trap (NET) formation and degradation plays a central role in the pathophysiology of inflammation, coagulopathy, organ damage, and immunothrombosis that characterize severe cases of COVID-19. Here, we discuss the evidence supporting a role for NETs in COVID-19 manifestations and present putative mechanisms, by which NETs promote tissue injury and immunothrombosis. We present therapeutic strategies, which have been successful in the treatment of immunο-inflammatory disorders and which target dysregulated NET formation or degradation, as potential approaches that may benefit patients with severe COVID-19.
© 2021. The Author(s).
Conflict of interest statement
MA, RB, GLB, CD, RdL, TH, AH, M Hoffmann, BH, MJK, JSK, JK, EK, PK, ML, AM, AAM, CM, NM, IM, LEM, TN, EN, IN, LGN, MZR, KR, PR-Q, M Schapher, CS, GS, H-US, JS, PS, KS, M Stürzl, PV, JvV, LV, MvK-B, CY, SY, and AZ have nothing to disclose. H-JA reports personal fees from AstraZeneca, personal fees from Boehringer, personal fees from Previpharma, personal fees from Secarna, personal fees from Inositec, personal fees from Novartis, personal fees from Bayer, personal fees from GSK, outside the submitted work. ME reports personal fees from CytoxM, personal fees from MPM Capital, non-financial support from Santhera, outside the submitted work. M Herrmann reports non-financial support from Neutrolis outside the submitted work; YK reports personal fees from Surface oncology, personal fees from Acer Therapeutics, outside the submitted work.
Figures




References
Publication types
MeSH terms
Grants and funding
- 4D Nanoscope/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- TRR241: B04/Deutsche Forschungsgemeinschaft (German Research Foundation)
- CRC1181: C03/Deutsche Forschungsgemeinschaft (German Research Foundation)
- K08 HL131993/HL/NHLBI NIH HHS/United States
- R01 HL150392/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

