Predictors of blood pressure response to ultrasound renal denervation in the RADIANCE-HTN SOLO study
- PMID: 34031548
- PMCID: PMC9287166
- DOI: 10.1038/s41371-021-00547-y
Predictors of blood pressure response to ultrasound renal denervation in the RADIANCE-HTN SOLO study
Abstract
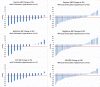
The blood pressure (BP) lowering response to renal denervation (RDN) remains variable with about one-third of patients not responding to ultrasound or radiofrequency RDN. Identification of predictors of the BP response to RDN is needed to optimize patient selection for this therapy. This is a post-hoc analysis of the RADIANCE-HTN SOLO study. BP response to RDN was measured by the change in daytime ambulatory systolic blood pressure (dASBP) at 2 months post procedure. Univariate regression was used initially to assess potential predictors of outcome followed by multivariate regression analysis. In the univariate analysis, predictors of response to RDN were higher baseline daytime ambulatory diastolic blood pressure (dADBP), the use of antihypertensive medications at screening, and presence of orthostatic hypertension (OHTN) whilst the presence of untreated accessory arteries was a negative predictor of response. Multivariate analysis determined that dADBP and use of antihypertensive medications were predictors of response to RDN with a trend for OHTN to predict response. Obese females also appeared to be better responders to RDN in an interaction model. RDN is more effective in patients with elevated baseline dADBP and those with OHTN, suggesting increased peripheral vascular resistance secondary to heightened sympathetic tone. These assessments are easy to perform in clinical setting and may help in phenotyping patients who will respond better to RDN.
© 2021. The Author(s).
Conflict of interest statement
MS has received grant support and personal fees from ReCor Medical. RES has received grant support and personal fees from ReCor Medical, Medtronic, and Ablative Solutions. AJK reports Institutional funding to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, ReCor Medical. In addition to research grants, institutional funding includes fees paid to Columbia University and/or Cardiovascular Research Foundation for speaking engagements and/or consulting. Personal: Consulting: Neurotronic; Travel Expenses/Meals from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. FM is supported by Deutsche Gesellschaft für Kardiologie (DGK), and Deutsche Forschungsgemeinschaft (SFB TRR219) and has received scientific support and speaker honoraria from Bayer, Boehringer Ingelheim, Medtronic and ReCor Medical. JD has received grant support from ReCor Medical, Medtronic, Boston Scientific, Abbott Vascular, Acist Medical, Astra Zeneca, Pie Medical, and Pulse Cath and has received personal fees from ReCor Medical, Medtronic, Acist Medical, Boston Scientific, Pie Medical, and Pulse Cath. JB has received grant support from ReCor Medical and Ablative Solutions. PL has received grant support and personal fees from ReCor Medical, Edwards, and Abbott and personal fees from Medtronic and Occlutech. PG has received grant support from Recor and Ablative Solutions. KS has received grant support and personal fees from ReCor Medical and Medtronic and grant support from CSI. NDLF has received grant support and personal fees from ReCor Medical. LCR has received personal fees and other support from ReCor Medical. AP has received personal fees from ReCor Medical, Medtronic, CVRx, Ablative Solutions, Dynamics, ROX Medical and grants from Medtronic, Ablative Solutions, ReCor Medical and CVRx. PJB reports institutional funding by the European Commission, Recor, Medtronic, Ablative Solutions, Fresenius and BBraun. MAW has received personal fees from ReCor Medical, Medtronic, Boston Scientific, and Ablative Solutions. ASPS has received personal fees from ReCor Medical, Medtronic and Philips. MJB has received personal fees from ReCor Medical and Medtronic. NCB is an employee of ReCor Medical and has multiple patents in renal denervation issued. LC is an employee of ReCor Medical. MA has received research grants from The French Ministry of Health, Quantum genomics and European H2020 program; has received grant support and non-financial support from ReCor Medical and Idorsia; and has received personal fees from CVRx. MDL has received personal fees from ReCor Medical, Medtronic, CVRx, Ablative Solutions, Vascular Dynamics, ROX Medical and Tarilan Laser Technologies and grants from Medtronic. All other authors declare no competing interests.
Figures
References
-
- Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–45. doi: 10.1016/S0140-6736(18)31082-1. - DOI - PubMed
-
- Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–70. doi: 10.1016/S0140-6736(17)32281-X. - DOI - PubMed
-
- Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–55. doi: 10.1016/S0140-6736(18)30951-6. - DOI - PubMed
-
- Böhm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–51. doi: 10.1016/S0140-6736(20)30554-7. - DOI - PubMed
-
- Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Lobo MD, et al. Six-Month results of treatment-blinded medication titration for hypertension control after randomization to endovascular ultrasound renal denervation or a sham procedure in the RADIANCE-HTN SOLO trial. Circulation. 2019;139:2542–53. doi: 10.1161/CIRCULATIONAHA.119.040451. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

