Shear-stress sensing by PIEZO1 regulates tendon stiffness in rodents and influences jumping performance in humans
- PMID: 34031557
- PMCID: PMC7612848
- DOI: 10.1038/s41551-021-00716-x
Shear-stress sensing by PIEZO1 regulates tendon stiffness in rodents and influences jumping performance in humans
Abstract
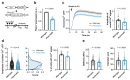
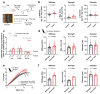
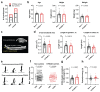
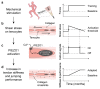
Athletic performance relies on tendons, which enable movement by transferring forces from muscles to the skeleton. Yet, how load-bearing structures in tendons sense and adapt to physical demands is not understood. Here, by performing calcium (Ca2+) imaging in mechanically loaded tendon explants from rats and in primary tendon cells from rats and humans, we show that tenocytes detect mechanical forces through the mechanosensitive ion channel PIEZO1, which senses shear stresses induced by collagen-fibre sliding. Through tenocyte-targeted loss-of-function and gain-of-function experiments in rodents, we show that reduced PIEZO1 activity decreased tendon stiffness and that elevated PIEZO1 mechanosignalling increased tendon stiffness and strength, seemingly through upregulated collagen cross-linking. We also show that humans carrying the PIEZO1 E756del gain-of-function mutation display a 13.2% average increase in normalized jumping height, presumably due to a higher rate of force generation or to the release of a larger amount of stored elastic energy. Further understanding of the PIEZO1-mediated mechanoregulation of tendon stiffness should aid research on musculoskeletal medicine and on sports performance.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
Shear mechanosensing drives tendon adaptation.Nat Biomed Eng. 2021 Dec;5(12):1409-1410. doi: 10.1038/s41551-021-00724-x. Nat Biomed Eng. 2021. PMID: 34031560 No abstract available.
References
-
- Magnusson SP, Langberg H, Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010;6:262–268. - PubMed
-
- Dickinson MH, et al. How animals move: an integrative view. Science. 2000;288:100–106. - PubMed
-
- Roberts TJ, Marsh RL, Weyand PG, Taylor CR. Muscular force in running turkeys: the economy of minimizing work. Science. 1997;275:1113–1115. - PubMed
-
- Wilson AM, Watson JC, Lichtwark GA. Biomechanics: A catapult action for rapid limb protraction. Nature. 2003;421:35–36. - PubMed
-
- Arampatzis A, Karamanidis K, Albracht K. Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J Exp Biol. 2007;210:2743–2753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

